Fig. 5. MD simulations of syntactically related IDPPs reveal interchain interaction forces that promote hysteresis.
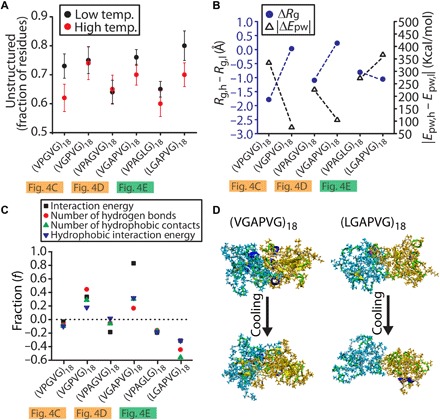
(A and B) Simulations of 18-mer, single IDPP chains at low (290 to 310 K) and high (350 to 390 K) temperatures for three IDPP pairs studied in Fig. 4 (C to E). (A) Fraction of residues that are part of unstructured motifs for single IDPP chains at high and low temperatures (see fig. S9 for all other structural motifs). (B) Temperature-dependent changes in radius of gyrations (Rg, black) and absolute peptide-water interaction energy (Epw, blue), expressed as a differential between values at high and low temperatures (ΔRg and ΔEpw). Dashed lines between data points for IDPPs in each pair are guides to the eye. (C and D) Simulations of two closely interacting 18-mer IDPP chains for each IDPP in Fig. 4 (C to E) to study interchain interactions in a model phase separated state. (C) Fraction of value changes (f) in interchain interaction quantities (see Supplementary Methods for details) after cooling these two chain “phase separated” systems to 290 K for 25 ns. f = (end value − initial value)/(initial value). (D) Snapshots from our two-chain simulations for an IDPP with marked hysteresis (VGAPVG)18 (Fig. 4D) and for an IDPP with negligible hysteresis (LGAPVG)18 (Fig. 4E). The production simulations were performed for 25 ns with a 2-fs time step.
