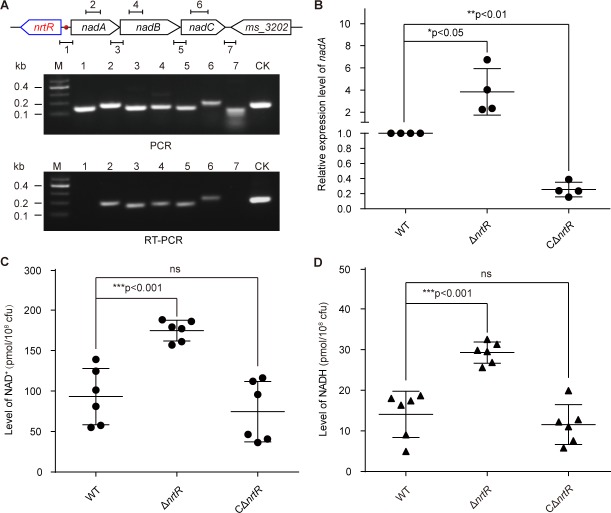
Figure 4. NrtR is a repressor for the nadABC operon that is responsible for NAD+ and NADH concentration in M. smegmatis.
(A) Genetic organization and transcriptional analyses of the nrtR and its neighboring de novo NAD+ synthesis genes. The arrows represent open reading frames, and the numbered short lines (1 to 7) represent the specific PCR amplicons that were observed in the following PCR and RT-PCR assays (in the bottom panels). PCR and RT-PCR were applied to analyze the transcription of the putative NAD+ de novo synthesis loci. The primer numbering was identical to that shown in the top panel. CK (control) denotes the 16S rDNA. (B) RT-qPCR analyses of nad operon expression in the wild-type strain and in the ΔnrtR mutant and nrtR complementary strains. RT-qPCR experiments were performed at least three times and the data were expressed as means ± standard deviations (SD). The p-value was calculated using one-way ANOVA along with Tukey's test. *p<0.05 and **p<0.01. Comparison of the intra-cellular level of NAD+ (C) and NADH (D) among the WT, ΔnrtR and CΔnrtR strains. Each dark circle or triangle represents an independent experiment. The data are shown as means ± SD. The statistical significance of differences among WT, ΔnrtR and CΔnrtR was determined by Student’s t test and by ANOVA with heterogeneous variances. ***p<0.001; ns, no significant difference.
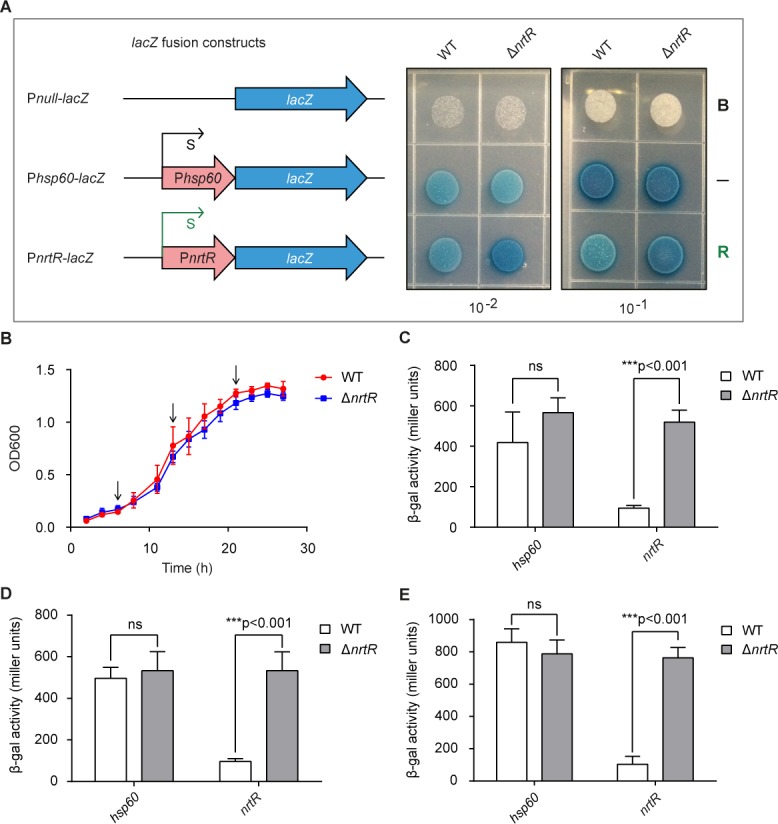
Figure 4—figure supplement 1. In vivo evidence that MsNrtR is an auto-repressor.