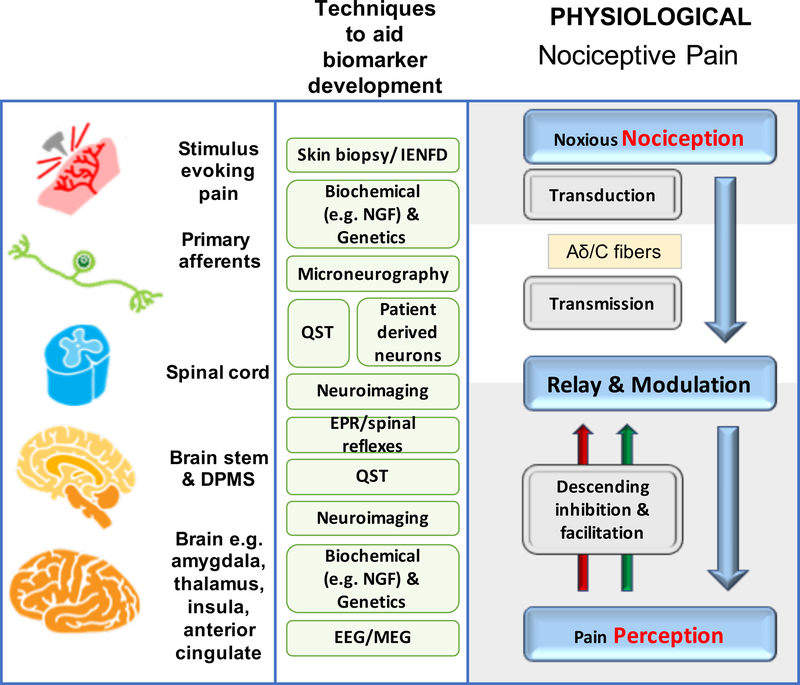
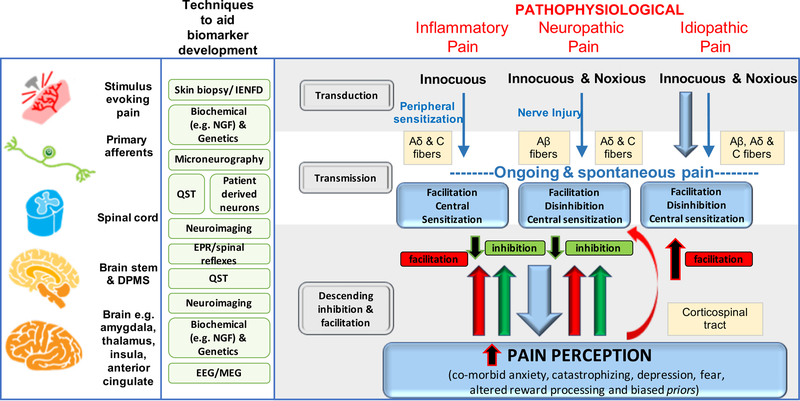
Figure 1a, b. Mechanisms of acute and chronic pain:
The normal physiological response to an acute noxious stimulus is depicted in black with the involvement of Aδ, and C fiber nociceptors transducing the input from the periphery to the superficial laminae of the dorsal horn of the spinal cord where they can be modulated. From the spinal cord, signals are relayed via regions in the brain stem to the brain where pain emerges as a perception and the sensory and emotional context and learning is applied to interpret and aid future avoidance of the stimulus. The major classes of chronic pain and the processes which are believed to lead to chronic pain in susceptible individuals are depicted in red. During inflammation, while the stimulus e.g. activated immune cells or a skin incision is present, there exists a status of peripheral sensitization (PS) characterized by erythema and tenderness to innocuous stimuli, typically heat. PS goes away once the peripheral pathology resolves. Stimuli activating nociceptors that are noxious, repeated and sustained e.g. following nerve injury, induce the process of central sensitization (CS) in the dorsal horn spinal cord. Initially CS is protective and enables the organism to avoid further injury due to a heightened awareness of its surroundings but at some point CS becomes pathological. CS produces pain in non-inflamed tissue by co-opting novel inputs e.g. Aβ fibers, thus mechanical pain is typical of CS and heat pain is more typical of peripheral sensitization. Recently it has been shown that a subset of corticospinal neurons (CSNs) known to originate in the primary and secondary somatosensory cortex and to directly innervate the spinal dorsal horn via CST axons can directly modulate normal and pathological tactile sensory processing in the spinal cord. Facilitation and descending inhibition are processes that occur due to different regions of the brain and brainstem inhibiting or activating (or even disinhibiting) nociceptive inputs to the spinal cord. The effect can be seen on both mechanical and heat sensations in different forms of chronic pain and an imbalance in this system (less inhibition, more facilitation) is a key mechanism, as are changes in the brain’s neurochemistry, structure and functional activity. Shown in green are the current methodologies that are used to define biomarkers at the particular levels of nociceptive and pain processing that apply to acute and chronic pain