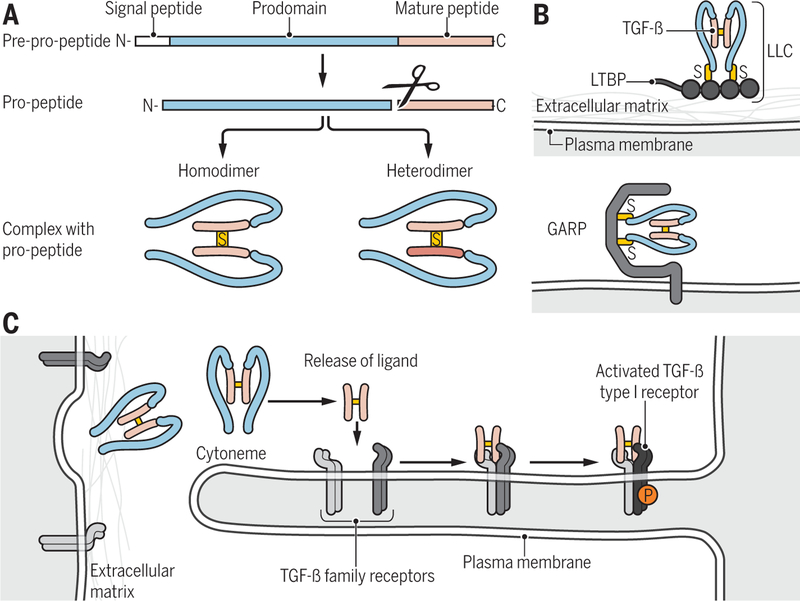
Figure 1. Ligand processing and presentation.
(A) TGF-β family proteins are synthesized as precursor molecules consisting of a signal peptide, a prodomain (termed latency-associated peptide, LAP, for TGF-β), and the mature polypeptide. After signal peptide removal, the precursor is further processed by proteolytic cleavage at basic residues, thus separating the prodomain from the mature polypeptide, which remain non-covalently associated. Concomitant, disulfide-linked dimerization of the mature polypeptides into mature homo- and heterodimeric proteins is shown. (B) Latent TGF-β complex can associate through disulfide bonding with LTBP into a large latent complex (LLC) that in turn associates with the extracellular matrix (top), or with the plasma membrane-associated GARP (bottom). (C) Cytoneme-associated activation of TGF-β family signaling. Long cytonemes extend from the cell body, and present TGF-β family receptors to ligand complexes. Binding of ligand to the receptors results in activation of the type I receptors (light to dark blue).
Credit: Veronica Falconieri Hays/Science Signaling