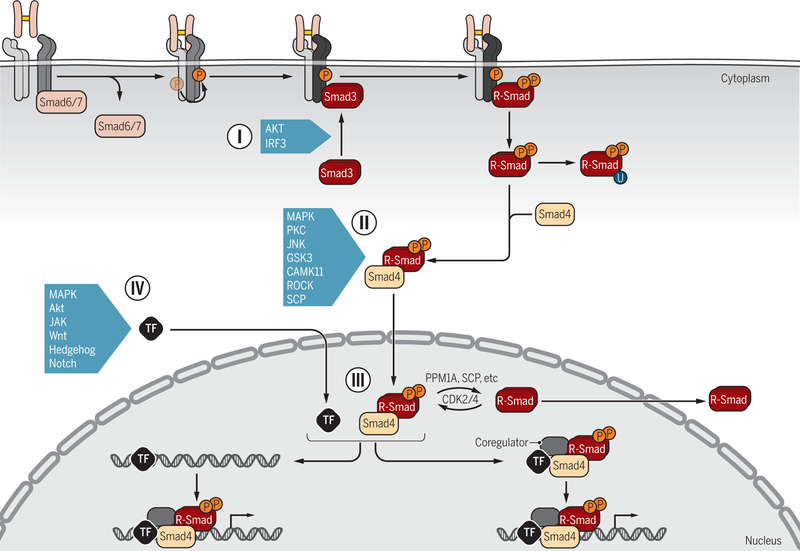
Figure 6. Signaling crosstalk through posttranslational control of Smad activation and functions.
R-Smad association with the receptors (I) is controlled by inhibitory Smad6 and/or Smad7, which prevent R-Smad access to the activated type I receptors (dark blue). Additionally, upon activation in response to various signaling pathways, AKT and IRF3 bind to Smad3 and thus attenuate Smad3 binding to activated type I receptors. Various signaling pathways that act through kinases target the linker regions of R-Smads for phosphorylation (II), with the possibility for further regulation by subsequent dephosphorylation, and thus control the subcellular localization, stability and function of Smads. Smads can also be poly-ubiquitylated, leading to degradation, and in some cases targeted linker phosphorylation is a prerequisite for subsequent poly-ubiquitylation and degradation. Some kinases and a phosphatase are listed as examples. In the nucleus (III), phosphorylation and dephosphorylation by kinases and phosphatases further regulate the Smad activities. Direct transcriptional activation or repression of target genes requires association of Smad complexes with DNA-binding transcription factors (TFs) and coregulators (IV). Smads have been shown to associate with a wide variety of TFs, depending on the signaling status of the cells and the targeted gene. Extensive signaling crosstalk occurs at the level of Smad-complex association with DNA-binding TFs, because they are also regulated by phosphorylation or other modifications in response to signaling pathways. Some examples are listed. Such crosstalk may occur before binding of Smad-TF complexes to regulatory gene sequences or after formation of the DNA-binding nucleoprotein complexes.
Credit: Veronica Falconieri Hays/Science Signaling