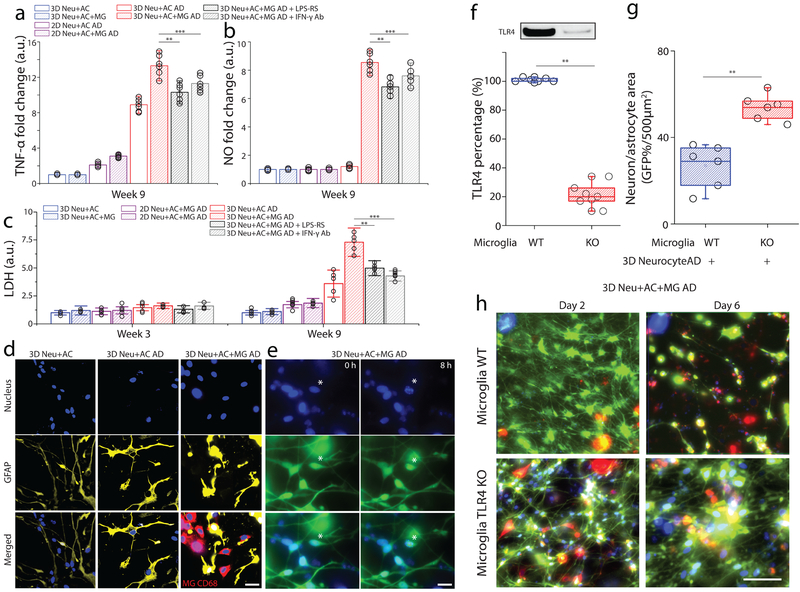
Figure 5. Assessment of neurotoxic neuron-glia interactions mediated by TLR4 and IFN-γ receptor.
In a tri-culture, 3D Neu+AC+MG AD express elevated levels of (a) TNF-α and (b) NO compared to the control (3D Neu+AC+MG and 2D/3D Neu+AC AD) and treatment using either TLR4 antagonist or blocking IFN-γ antibody reduced the concentrations of TNF-α and NO (R-square=0.9024, F(DFn, DFd)=1.061(4, 10), F= 23.11), All experiments were repeated ≥3 times, Two-way ANOVA. (c) In the 3D Neu+AC+MG AD after 9-week of differentiation, neurotoxic interactions were mimicked by LDH concentrations (R-square=0.7104, F (1, 14) = 15.65); the damage was attenuated through inhibition using a TLR4 antagonist and a blocking IFN-γ antibody (f, R-square=0.8954, F (1, 14) = 22.61). (d) Immunostaining of the astrocyte marker GFAP (yellow) and microglial CD68 (red) demonstrated the activation of astrocytes and microglia in 3D Neu+AC AD and 3D Neu+AC+MG AD compared to 3D Neu+AC. (e) Apoptotic cells with fragmented nuclei observed after 8h in 3D Neu+AC+MG AD. (f) Immunoblot and quantification of proteins isolated from WT and TLR4 knock-down microglia. Fold change was determined by normalizing TLR4 band intensity to Actin band (R-square=0.8934, F (1, 3) = 21.65). Reduced neuron+astrocyte loss was (g) measured with GFP-overlaying surface area (R-square=0.8240, F (1, 3) = 19.25) and (h) visualized with fluorescent microphotographs in 3D Neu+AC AD with TLR4-KO microglia. All experiments were repeated ≥3 times. Two-way ANOVA test; Statistical significance is denoted by # IFN-γ vs IFN-γ + Aβ, † Aβ vs IFN-γ + Aβ, #* IFN-γ + Aβ vs IFN-γ + Aβ + TLR4 Ab, †* IFN-γ + Aβ vs IFN-γ + Aβ + LPS-RS P<0.0002 and **p<0.001, ***p<0.0001with numberwell = 5 in (a, b), numberdevice = 5 in (c, d, e), respectively. All parameters are presented as the mean ± SEM. Scale bars: 10 μm in (d), 5 μm in (i) 40 μm in (i), respectively.