Fig. 3.
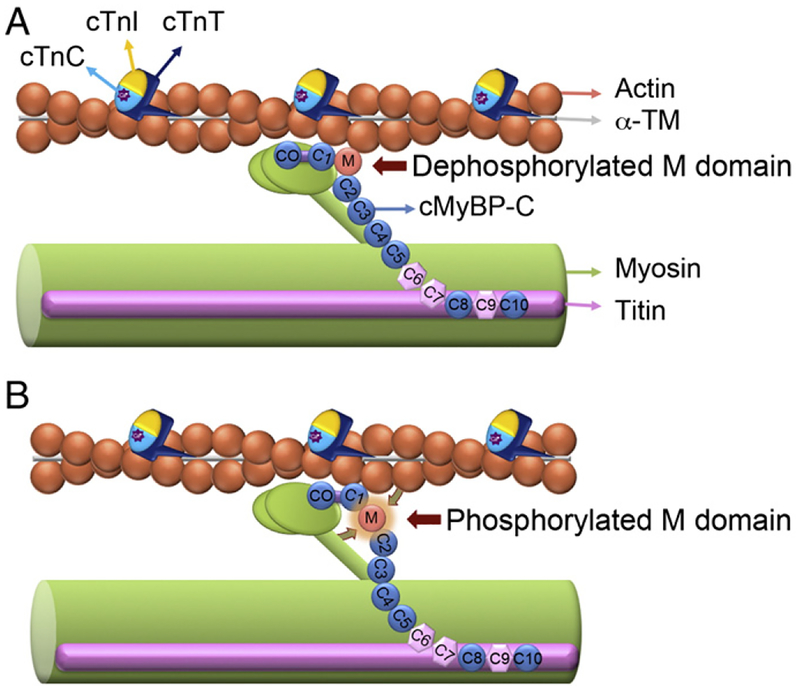
Dynamic phosphorylation of cMyBP-C’s M domain influences myosin–actin interactions. cMyBP-C facilitates myosin–actin interactions by tethering the fibers together and regulating the ability of the myosin head to bind to the actin filament during cross-bridge cycling. cMyBP-C interacts with the LMM of myosin at the C10 domain and titin at domains C8–C10. The C1-M-C2 domains have been shown to bind to myosin and actin. The capacity of these domains to interact is critical to overall sarcomeric integrity and function. The phosphorylatable M domain facilitates the role of cMyBP-C in cross-bridge formation. In the absence of M domain phosphorylation, the C1-M-C2 domains are tightly bound to myosin S2 region (A), whereas when M domain phosphorylated, C1-M-C2 domains and M domain release their interaction with myosin S2 region and actin, respectively, and changes in the thick filament orientation occur (B). C1 domain binds to actin irrespective of the M domain phosphorylation state. The dynamic phosphorylation of the M domain provides a point of control where the ordering of myosin heads and changes in force production can be regulated. Thin filament proteins include actin, α-TM, cTnI, cTnT and calcium (Ca2+)-binding protein cTnC are shown.