Abstract
Introduction
Although natural killer (NK) are major cells used to treat cancer patients, recent clinical trials showed that NK92 cells can be also used for the same purpose due to their high anti-tumor activity. Here, we examined whether these cells might be inflammatory due to the release of interleukin-1β (IL-1β), and whether the anti-inflammatory molecules dimethyl fumarate (DMF), or monomethyl fumarate (MMF) impair this activity.
Methods
NK92 cells were examined for the synthesis and release of IL-1β utilizing RT-PCR and ELISA assay, respectively. The expression of hydroxy-carboxylic acid receptors (HCA)1, HCA2 and HCA3 was detected by immunoblotting, flow cytometry, immunofluorescence and RT-PCR assays. The activation of caspase-1 and Gasdermin D (GSDMD) was evaluated by immunoblot assay. Pyroptosis was demonstrated by immunofluorescence imaging. Expression of DNA methyltransferases (DNMTs) mRNA was determined by whole transcriptome and immunoblot analyses.
Results
LPS-induced the release of IL-1β from NK92 cells, whereas DMF or MMF inhibited this induction. The effect of these drugs was due to inhibiting the conversion of procaspase-1 into active caspase-1. NK92 cells highly expressed GSDMD, a pyroptotic-mediated molecule. However, LPS induced the distribution of GSDMD into the cell membranes, corroborated with the presence of pyroptotic bodies, an activity that was inhibited by DMF or MMF. These molecule also inhibited the generation of GSDMD through DNMT-mediated hypermethylation of the promoter region of GSDMD gene. These results were supported by increased expression of DNMTs mRNA as determined by whole transcriptome analysis.
Discussion
Our results are the first to show that NK92 cells utilize GSDMD pathway to release IL-1β. Further, DMF and MMF which were previously shown to enhance NK cell cytotoxicity, also inhibit the inflammatory effects of these cells, making them most suitable for treating cancer patients.
Keywords: pyroptosis, gasdermin D, NK cells, IL-1β, dimethyl fumarate, monomethyl fumarate
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Natural killer (NK) cells are highly anti-tumor effector cells and are used for the treatment of cancer patients (https://clinicaltrials.gov/ct2/results?term=nk+cells+and+cancer&pg=9). To compensate for the inability to generate large numbers of NK cells for cellular therapy, investigators utilize human NK cell lines for this purpose. NK92 cells were isolated from NK cell lymphoma patient, and have been used for treating cancer patients due to their highly anti-tumor activity.1,2 It is also because these cells can be easily expanded in vitro as well as their ease of manipulation. In addition to their high anti-tumor cytolytic activity, NK cells also have immunoregulatory activity secreting various cytokines and chemokines that regulate various aspects of the adaptive immune system.3 Although IFN-γ is the prototype cytokine secreted by NK cells,4 we previously reported that IL-2-activated NK cells secrete several cytokines, among them is IL-1β, but the mechanism of IL-1β exocytosis is not known.5
IL-1β belongs to a large family of molecules, including IL-1α, IL-18, IL-33, IL-36, and IL-38. Among many of its pro-inflammatory effects, IL-1β facilitates the activation of innate immune cells such as monocytes.6 As an inflammatory cytokine, IL-1β induces tumor angiogenesis,7 among many other functions. The value of therapeutically targeting IL-1β pathway with human IL-1β receptor antagonist or the anti-IL-1β antibodies has increasingly been explored in patients with malignancies.8 Therefore carefully designed studies are necessary to target IL-1β pathway in each of the specific disease settings.
Monocytes release IL-1β by a process that activates inflammasome.9 Recently, a phenomenon known as pyroptosis was implicated in its release. This phenomenon is a form of cell death where the cells generate pyroptotic bodies concomitantly with flattening the cytoplasm and the formation of pores in the plasma membranes.10,11 The release of IL-1β is mediated by a newly described molecule called gasdermin-D (GSDMD),12 which is the most important player that mediates pyroptosis.13,14 Before IL-1β is released, it must be synthesized intracellularly, and as indicated, it is widely accepted that inflammasomes are important for the production of this cytokine. There are several types of inflammasomes including canonical and non-canonical forms.15 Canonical inflammasome consists of the sensor NOD-like receptor pyrin domain containing 3 (NLRP3), the adaptor protein apoptosis-associated speck-like protein containing CARD domain (ASC), and the procaspase-1 which upon activation is converted into the effector caspase-1 that generates IL-1β from its precursor. The non-canonical forms include those that consist of caspase 4 and 5 or 11 in mice. In this work, we sought to examine in details the pathways of IL-1β release from NK92 cells
Dimethyl fumarate (DMF) is a drug approved for treating multiple sclerosis patients under the name Tecfidera (Biogen). This drug has pleiotropic anti-inflammatory activities and can be used to treat various disorders.16 On the other hand, monomethyl fumarate (MMF) is not a drug but it possesses multiple activities, including the activation of anti-tumor effector cells.17,18 DMF or MMF have been used in this study to investigate their anti-pyroptotic effects in NK92 cells.
Materials And Methods
Reagents And Cell Culture Treatment
NK92 cells “CRL-2407” and the monocytic cell line U-937 “CRL-1593.2” were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI 1640 medium supplemented with 4.5 g/l glucose, 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mM L-glutamine, 1% nonessential amino acids, 71.5 µM 2-mercaptoethanol and 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO, USA). Unconjugated polyclonal antibodies against HCA1, HCA3 were obtained from Abcam (Cambridge, UK), and a polyclonal antibody for HCA2 was obtained from Invitrogen (Carlsbad, CA, USA). Anti-rabbit secondary antibody tagged with Alexa 488 was obtained from Invitrogen. MMF, DMF, and LPS were obtained from Sigma-Aldrich. For each in vitro experiment, NK92 cells (1 × 106/mL), or U937 cells were incubated with 100 μM DMF or 100 μM MMF or 10 μg/mL LPS with RPMI culture medium primed with 200 IU/mL IL-2 for 24 h at 37°C in 5% CO2 incubator. The 100 μM dose of DMF or MMF was found to be optimal in various biological activities of NK92 cells.17
Quantitative Real-Time Reverse Transcription (RT)-PCR
Total RNA or DNA was extracted from cell lysates using AllPrep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s protocol. RT-PCR was performed using 1 μL of cDNA. Primers for HCA1 (Hs02597779), HCA2 (Hs02341584), and HCA3 (Hs02341102) were assessed using Taqman Gex Master Mix and StepOne System (Applied Biosystems, USA). Specific primers for IL-1β, GSDMD are shown in Table 1. Promega GoTaq® qPCR Master mix(Promega, Madison, WI, USA) and Qiagen Rotor-gene qPCR machine (Qiagen) were used for SyberGreen qPCR. Expression levels of target genes were normalized to GAPDH expression.
Table 1.
Sequence Of Primers Used In This Study
| (a) List And Sequences Of RT-PCR Primers | |||||
|---|---|---|---|---|---|
| Gene | F/R | Sequence (5ʹ > 3ʹ) | Melting Temperature (°C) | Product Size (bp) | |
| IL-1β | F R |
AGCTACGAATCTCCGACCAC CGTTATCCCATGTGTCGAAGAA |
61.1 60.1 |
186 | |
| HCA1 | F R |
CTCATTGTGGCCTTTGTGCT GAATGTCCCCAAAAGCCCAG |
59.0 59.1 |
196 | |
| HCA2 | F R |
GCTGTGTGTTCCGAGATGAC CCAAACTTCCAGTCCCAACG |
58.9 59.0 |
235 | |
| HCA3 | F R |
TGCCAAGATCAAGAGAGCCA GAAGTTGGGAAAGGATGGGC |
59.0 58.8 |
244 | |
| GSDMD | F R |
GTGTGTCAACCTGTCTATCAAGG CATGGCATCGTAGAAGTGGAAG |
60.5 60.7 |
94 | |
| (b) List and sequences of primers used in MSP | |||||
| Gene | F/R | M/U | Sequence (5ʹ > 3ʹ) | Melting Temperature (°C) | Product size (bp) |
| GSDMD | F R |
M | ATTTTGTATACGTAGTAGAAGGGACG TTACGAAAATCGACGAAACG |
58.3 58.8 |
98 |
| GSDMD | F R |
U | TGTATATGTAGTAGAAGGGATGTGG AAAAATCAACAAAACACCCT |
56.5 53.8 |
90 |
Abbreviations: F, forward; R, reverse; M, methylated; U, unmethylated.
Methylation-Specific (MSP)-PCR
To analyze the presence of CGI in the promoter region of GSDMD gene, we used a public database available via the University of California Santa Cruz Genome Browser (http://genome.ucsc.edu/) on GRCh38/hg38 assembly (Genome Reference Consortium). Fully methylated and fully unmethylated control DNAs were purchased from Qiagen. A 2 μg of genomic DNA (gDNA) was treated with EpiTect Bisulfite Kit (Qiagen). MSP was conducted using 1 μL of the sodium bisulfite-treated DNA, primers specifically designed for methylated and unmethylated DNA sequence of the promoter region of GSDMD gene (Table 1), Promega GoTaq® qPCR Master mix (Promega) and Qiagen Rotor-gene qPCR machine (Qiagen) were used. DNA methylation levels were calculated as previously described.19
Western Blot Analysis
NK92 cells or U937 cells were lysed using Laemmli lysis buffer (Sigma-Aldrich). Blots were prepared and then blocked with 5% dry milk solution in TBST for 1 h. Primary antibodies to HCA1, HAC2, HCA3, gasdermin-D, DNMT3A, DNMT3B or Caspase-1 (Abcam, Cambridge, UK) were used. HRP conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Cell Signaling Technology, Danvers, MA, USA), were diluted in fresh 5% dry milk in TBST solution and incubated with the blots for 1 h at room temperature. HRP was detected using BioRad ECL Western blotting detection reagent (BioRad, Hercules, CA, USA). Primary antibody for Actin (Cell Signaling Technology) was used to confirm loading equality.
Flow Cytometric Analysis
NK92 cells were fixed with 70% ethanol and were labeled with primary antibodies for 1 h at 4°C, washed twice with PBS and labeled with the secondary goat anti-rabbit IgG labeled with the Alexa 488 at 1:800 dilution, incubated for 40 min at 4°C and then washed twice with PBS and acquired on Accuri C6 or BD FACSAria III flow cytometer (BD Biosciences, San Jose, CA, USA).
Confocal And Fluorescence Microscopy Analysis
NK92 cells untreated or treated with 100 μM DMF, 100 μM MMF, or 10 μg/mL LPS for 24 h were fixed by adding ice-cold 70% ethanol drop by drop and incubated at 4°C overnight. Fixed cells were washed with PBS twice and suspended in the FACS Buffer (2% BSA in PBS with Sodium Azide). Cells were incubated at 4°C for 2 h with the respective primary antibodies to HCA1, HAC2, HCA3, or gasdermin-D (Abcam). The samples were washed with FACS buffer twice and were labeled with respective secondary antibodies tagged with Alexa 488 for 45 min. The cells were placed on the slides using the CytoSpin (Thermo Fisher Scientific, Waltham, MA, USA). Slides were removed from the CytoSpin and the mounting media with ProLong gold antifade mountant with DAPI (Invitrogen, Carlsbad, CA, USA), was added to stain the nucleus. Slides were then observed under either confocal microscope (A1R Confocal Laser Microscope System, Nikon Inc., Tokyo, Japan) or fluorescence microscope (Olympus-BX43, Olympus Life Science, Waltham, MA, USA).
Enzyme-Linked Immunosorbent (ELISA) Assay
NK92 cells were incubated with 100 μM DMF, 100 μM MMF, DMSO, or 10 μg/mL LPS along with 200 IU/mL IL-2 for 24 h at 37°C in 5% CO2 incubator. After incubation, the supernatants were collected and stored at −80°C until further analysis. Levels of IL-1β were measured using human IL-1 beta ELISA kits (Abcam) according to the manufacturer protocol, in a BioTek PowerWave XS plate reader (Winooski, VT, USA). The standard curves and concentrations were calculated using Gen5 Data Analysis Software (BioTek Instruments).
Whole Transcriptome Analysis
RNA extracted from NK92 cells incubated with either 100 μM DMF, 100 μM MMF, DMSO, or 10 μg/mL LPS were analyzed for whole transcriptome profiling using targeted whole RNA-seq with AmpliSeq whole transcriptome on S5 system (Thermo Fisher Scientific). The targeted RNA-seq library was prepared using Ion AmpliSeq transcriptome human gene expression kit (Thermo Fisher Scientific) which is designed to profile over 21,000 distinct human RNA targets using a highly multiplexed amplification method. Each amplicon represents a unique targeted gene. The average size of each amplicon is ~150 bp. The RNA samples were quantified and ~30ng of Turbo DNase treated RNA was used to generate a barcoded cDNA library using SuperScript VILO cDNA synthesis kit (Invitrogen, Life Technologies Corp., Pleasanton, CA, USA). The cDNA library was amplified using Ion Ampliseq gene expression core panel technology to accurately maintain expression levels of all the targeted genes. The amplified cDNA libraries were quantified using Ion Taqman library quantitation kit (Applied Biosystems, (Life Technologies Corp, Austin, TX), and the libraries were further diluted to 100 pM and pooled equally with three individual samples per pool. The pooled libraries were amplified using emulsion PCR on Ion One Touch2 instruments (OT2) and enrichment was done on Ion One Touch ES following manufacturer’s instruction. Prepared template libraries were then sequenced on Ion S5 XL Semiconductor sequencer using Ion 540 Chip (Life Technologies Corporation, Carlsbad, CA).
Bioinformatics Analysis
RNA-seq data was analyzed using the Ion Torrent Software Suite version 5.4. Alignment was carried out using the Torrent Mapping Alignment Program (TMAP). TMAP is optimized for Ion Torrent sequencing data for aligning the raw sequencing reads against reference sequence derived from hg19 (GRCh37) assembly. To maintain specificity and sensitivity, TMAP implements a two-stage mapping approach. First, four alignment algorithms, BWA-short (BWA, http://bio-bwa.sourceforge.net), BWA-long, SSAHA, and super-maximal exact matching were employed to identify a list of candidate mapping locations. A further alignment process is performed using the Smith-Waterman algorithm,20 to find the final best mapping. Raw read counts of the targeted genes were performed using samtools (samtools view –c –F 4 –L bed_file bam_file). The quality control including the number of expressed transcripts is checked after Fragments per Kilobase Million (FPKM) normalization. Differentially Expressed Gene (DEG) analysis was performed using R/Bioconductor package DESeq2 with raw read counts from RNASeq and AmpliSeq. Read count normalization was performed using the regularized logarithm (rlog) method provided in DESeq2. Genes with less than ten normalized read counts were excluded from further analysis. DEG determination was carried out using LIMMA package (bioconductor.org, https://www.ncbi.nlm.nih.gov/pubmed/25605792). The fold change was calculated by comparing each of the drugs and LPS treated NK cells with vehicle.
Statistical Analysis
Student’s t-test was used to evaluate the significant differences between groups and between samples. Microsoft Excel 2018 (Microsoft Office, 2018) was used for all statistical calculations. Between two to three independent replicates were assessed in each experiment, and pooled data were presented as mean ± SEM. Quantification analysis of Western blotting data was performed using ImageJ software. A p value of <0.05 was considered significant.
Results
DMF Or MMF Inhibits LPS-Mediated IL-1β Production And Release From NK92 Cells
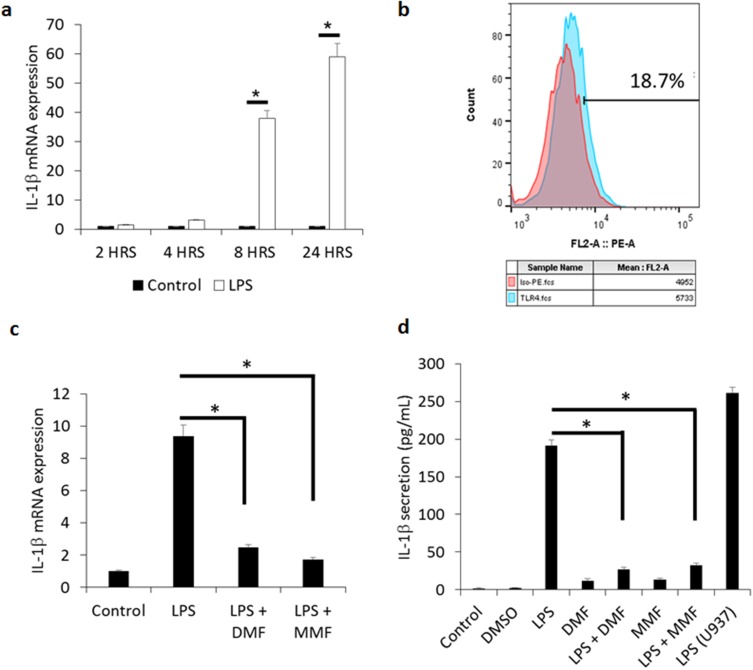
We previously reported that IL-1β is produced in moderate intensity by human IL-2-activated NK cells.5 To demonstrate whether NK92 cells may produce this cytokine, we measured its mRNA level in these cells. We observed about ~40 fold increase in IL-1β mRNA expression after treatment for 8 h with 10 μg/mL of LPS, and about ~60 fold increase after 24 h incubation, as compared to untreated cells (Figure 1A). LPS is known to activate NK cell cytotoxicity,21 promote CD56+ NK cell proliferation,22 and induce the release of IFN-γ from human NK cells,23 but the expression of receptors for LPS on NK cells was not studied in these reports. Here, we observed that between 12–20% of NK92 cells express TLR4 (Figure 1B), supporting the results of others showing expression of TLR4 in murine NK cells.24
Figure 1.
DMF and MMF inhibit the production and release of LPS-mediated IL-1β release from NK92 cells. (A) Time-dependent treatment of NK92 cells with 10 μg/mL LPS upregulates the expression of IL-1β mRNA. *P values (<0.01) compare the mRNA expression in LPS-activated cells (white columns) versus the background control (black columns). (B) Flow cytometry showing representative experiment of the binding of isotype control antibody (red color) and antibody against TLR4 (blue color). Percentages of positive cells are shown. (C) Comparison of mRNA expression of IL-1β among LPS-stimulated cells and those treated with LPS plus DMF or MMF. *P<0.01. (D) LPS-induced IL-1β release and its inhibition by DMF or MMF, as detected by ELISA assay. NK92 cells were incubated with 10 μg/mL LPS either alone or in the presence of 100 μM DMF or MMF in media containing 200 IU/mL IL-2 for 24 h. *P values (<0.01) compare IL-1β release in LPS-activated cells vs cells incubated with LPS plus DMF or MMF. U937 cells treated with 10 μg/mL LPS for 24 h were used as a positive control in these experiments.
Pretreatment of NK92 cells for 24 h with 100 μM DMF or MMF significantly inhibited LPS-induced mRNA expression of IL1β (P<0.01, Figure 1C). Upon examing the levels of IL-1β secreted by NK92 cells, we demonstrated that 24 h treatment with LPS induced the release of mature IL-1β from these cells. Next, we evaluated the effect of DMF or MMF on the release of IL-1β from LPS-treated NK92 cells, and observed that 24 h treatment with these compounds significantly reduced LPS-mediated IL-1β release from NK92 cells, when compared to control untreated cells (P<0.01, Figure 1D). DMF alone or MMF alone did not enhance the release of this cytokine. As a positive control, the U937 cell line known to have inflammasomes were used. U937 cells highly released IL-1β after incubating with 10 μg/mL of LPS (Figure 1D). Further, LPS induced IL-1β mRNA expression in these cells, whereas DMF or MMF impaired this activity (*P<0.01, Supplementary Figure 1), supporting those observed in NK92 cells,
NK92 Cells Express Receptors For DMF/MMF
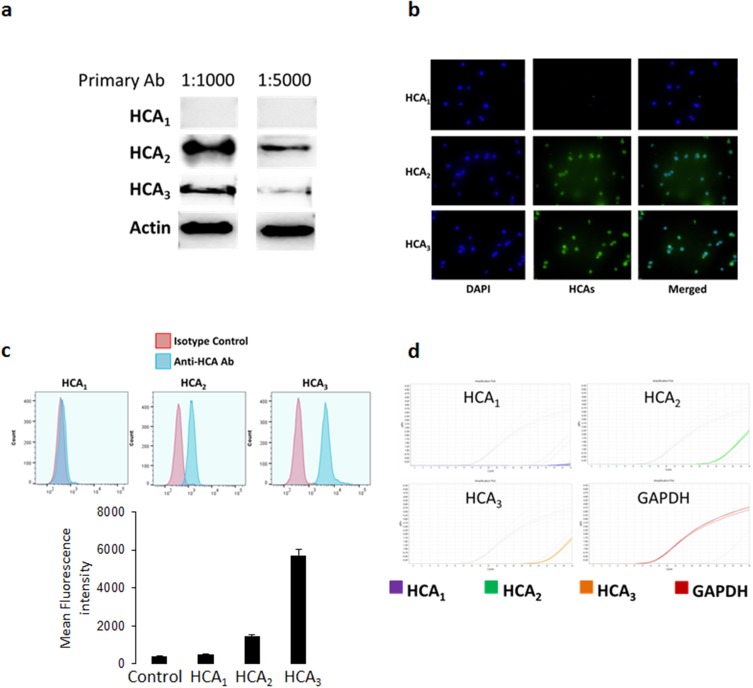
To further understand the pathways involved in the inhibitory effects of DMF and MMF, we investigated the presence of specific receptors for these ligands in NK92 cells. It has been previously reported that MMF mediated its therapeutic effects via binding hydroxy-carboxylic acid receptors (HCA).25 Using several experimental assays we demonstrated that NK92 cells express two of the three main members of the HCA receptor family. Western blotting results using 1:1000 or 1:5000 dilution of the antibody reveal that NK92 cells only expressed HCA2 and HCA3, but not HCA1 (Figure 2A). Similarly, immunofluorescence results show that NK92 cells expressed HCA2 and HCA3 (Figure 2B). We also demonstrate the presence of HCA2 and HCA3 by flow cytometry, showing that more than 90% of NK92 cells were stained for HCA2 and HCA3 but not HCA1 (Figure 2C). PCR data confirmed that NK92 cells express HCA2 and HCA3 only (Figure 2D).
Figure 2.
Expression of HCA receptors in NK92 cells. (A) Western blotting was performed on NK92 cell lysates incubated with primary antibodies for HCA1, HCA2, and HCA3 at two different concentrations (1:1000 and 1:5000). (B) Immunofluorescence staining of NK92 cells with specific antibodies for each HCA receptors. Green fluorescence showing binding of the anti-HCA antibodies, whereas blue fluorescence (DAPI staining) shows nuclear staining of the cells, and merged figures showing two images superimposed to each other for each HCA receptor. (C) Flow cytometry showing the expression of HCA1, HCA2, and HCA3. Red histograms showing the binding of the isotype control antibody, whereas blue histograms showing binding of the anti-HCA antibodies. (D) PCR data showing the mRNA expression of HCA receptors in NK92 cells. GAPDH mRNA expression was used as an internal control.
DMF Or MMF Treatment Inhibits LPS-Activated Conversion Of Procaspase-1 To Caspase-1 In NK92 Cells
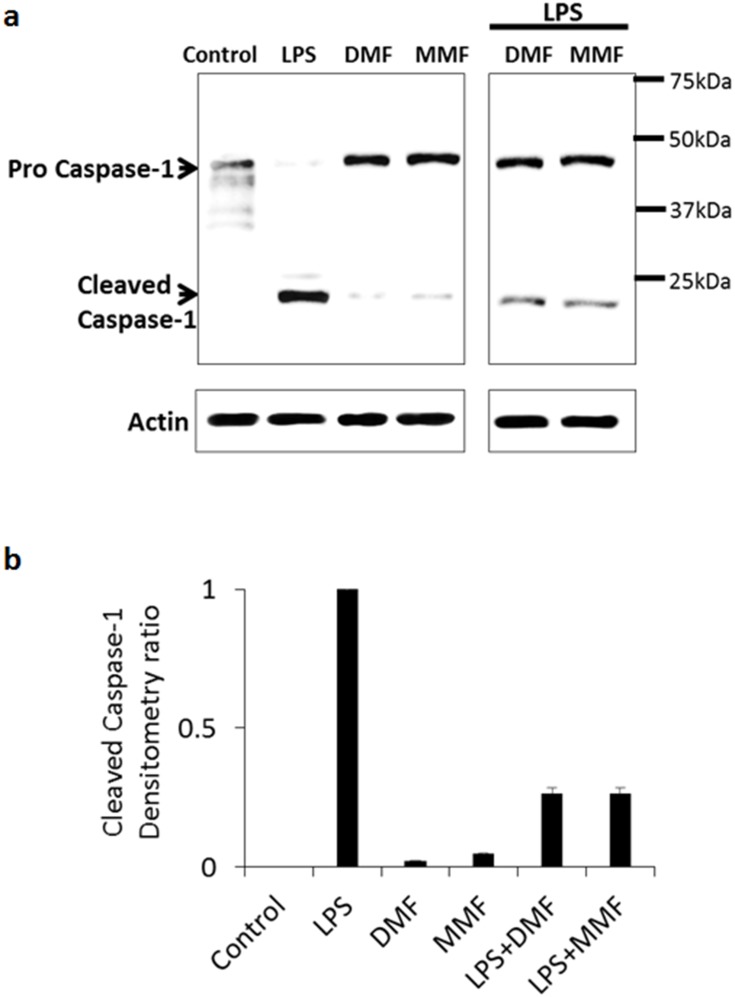
Secretion of IL-1β requires processing and conversion of pro-IL-1β into active IL-1β, mainly dependent on the activation of caspase-1. Purified LPS can induce the synthesis, processing, and release of IL-1β from human monocytes.26 However, to date, no such evidence exists in NK cells. To investigate this issue, we measured the expression and conversion of procaspase-1 to caspase-1 in NK92 cells by immunoblot analysis. Our results indicate that LPS treatment of NK92 cells induced the conversion of procaspase-1 into cleaved active-caspase-1 (Figure 3A). DMF or MMF did not induce this conversion (Figure 3A). However, when combined with LPS, these molecules significantly reduced the conversion of procaspase-1 into active caspase-1 as determined by immunoblot (Figure 3A), and by densitometry analysis (P<0.01, Figure 3B). Actin was used as a housekeeping gene.
Figure 3.
DMF or MMF treatment impairs LPS-activated conversion of procaspase-1 to caspase-1 in NK92 cells. (A) Treatment of NK92 cells with 10 μg/mL LPS for 24 h induced the conversion of procaspase-1 (45kDa) into cleaved subunits of active caspase-1 (22kDa and 12kDa, respectively). Combined treatment of LPS with DMF or MMF showed a reduction in the conversion of procaspase-1 into cleaved active-caspase-1. NK92 cells treated with100 μM DMF or MMF did not induce this conversion. (B) Densitometry calculation of the conversion of procaspase-1 into caspase-1. Actin was used as an internal control.
DMF Or MMF Reduces GSDMD Expression
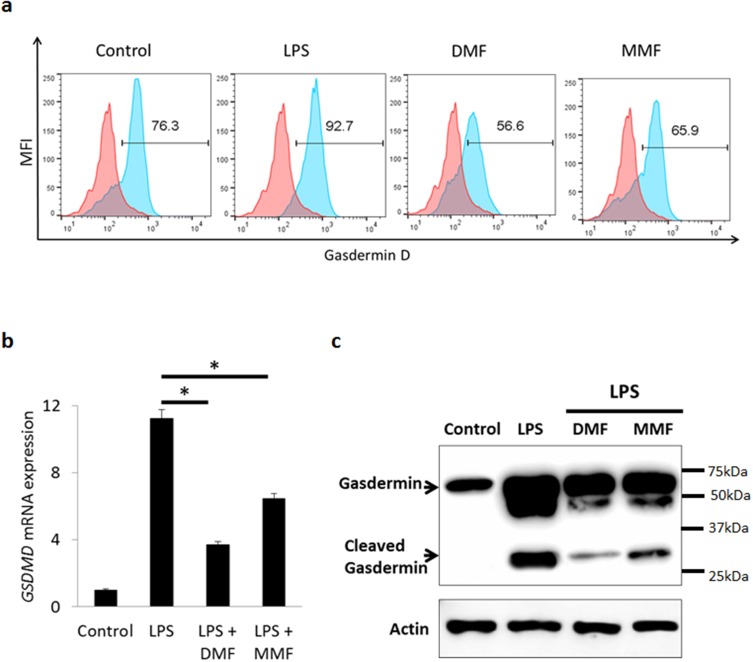
Recently, it has been reported that GSDMD is a component of inflammasomes, responsible for membrane pore formation via pyroptosis leading to secretion of matured IL-1β.10–14 To investigate this issue, we sought first to determine whether GSDMD is present in NK92 cells. Flow cytometric analysis demonstrates that more than 75% of untreated NK92 cells express this molecule (Figure 4A). The percentages of GSDMD expressing NK92 cells were increased upon LPS treatment but reduced after DMF or MMF treatment (Figure 4A). Further, we examined the mRNA level in cells treated with LPS or with the two molecules. Results in Figure 5B demonstrate that LPS treatment highly increased the mRNA levels of GSDMD, but this was significantly reduced upon DMF or MMF treatment for 24 h (Figure 4B).
Figure 4.
DMF or MMF treatment inhibits LPS-activated expression and activation of gasdermin-D (GSDMD) in NK92 cells. (A) Flow cytometry data showing an increase in the percentages of GSDMD positive cells after LPS treatment, but not post DMF or MMF treatment (B) RT-PCR data showing the mRNA expression of GSDMD gene expression. Cells were either left untreated (Control), treated with 10 μg/mL LPS or LPS plus 100 μM of either DMF or MMF in the presence of 200 IU/mL IL-2. *P<0.01 comparing the gene expression of GSDMD in the presence of LPS vs in the presence of LPS plus DMF or MMF. (C) Cleaving of GSDMD into active GSDMD in untreated (Control) NK92 cells, or cells incubated with LPS, DMF or MMF.
Figure 5.
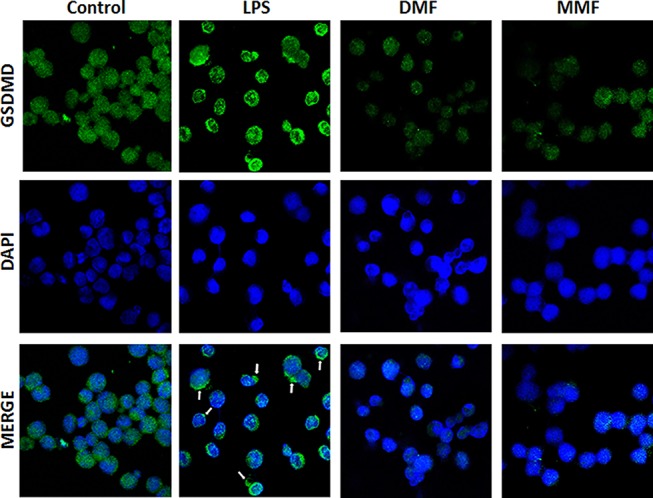
Confocal microscopyimages showing the expression and translocation of GSDMD. NK92 cells were either left untreated (Control) or incubated with 10 μg/mL LPS in the absence or presence of 100 μM of either DMF or MMF, and in the presence of IL-2. DPI was used to stain the nuclei. Arrows indicate pyroptotic bodies after treatment of NK92 cells with LPS.
Upon activation, the inflammatory caspases are known to cleave GSDMD, releasing the N-terminal p30 domain from the auto-inhibitory C-terminal p20 domain. Activated GSDMD then binds to phospholipids in the plasma membrane and forms large oligomeric pores releasing cellular contents, such as inflammatory cytokines. Here we demonstrate that LPS induced the cleavage of GSDMD into two components, whereas pretreatment with DMF or MMF for 24 h reduced the activation/cleavage of GSDMD induced by LPS (Figure 4C). As a control, LPS also induced GSDMD mRNA in U937 cells, and this activity was inhibited by pre-treating the cells with DMF or MMF (Supplementary Figure 2).
LPS Induces Pyroptosis In NK92 Cells
The confocal microscopic evaluation confirms that NK92 cells express GSDMD, but this molecule was unevenly distributed among the cytoplasm and the plasma membranes (Figure 5, Control). In contrast, incubation with LPS induced the translocation of this molecule towards the plasma membranes of these cells, corroborated with the appearance of several pyroptotic bodies in the membranes of these cells where GSDMD accumulate (white arrows, Figure 5, LPS). On the other hand, DMF or MMF reduced the intensity and the expression of LPS-induced GSDMD in these cells (Figure 5).
DMF Or MMF Induces DNMT-Mediated Methylation-Silencing Of GSDMD
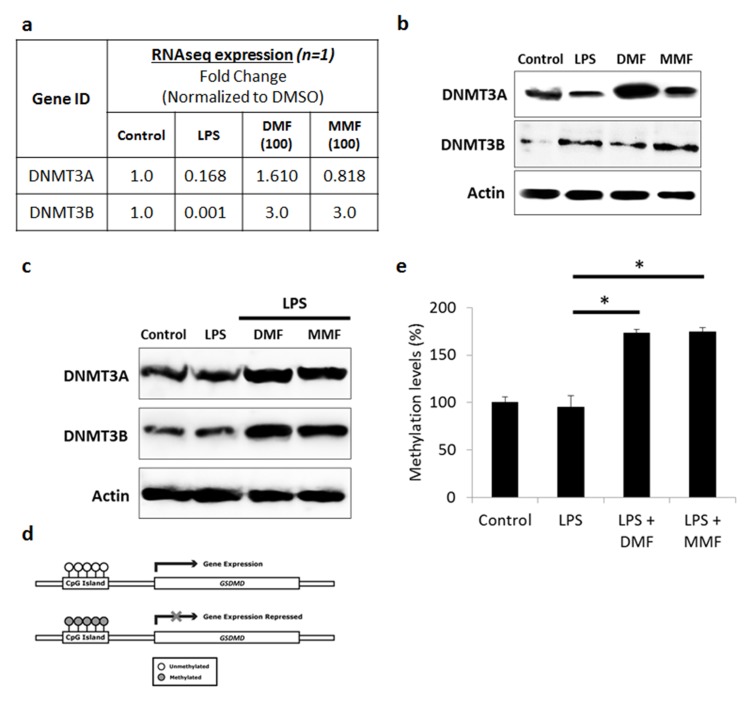
Our search through the UCSC genome browser determined the presence of CpG island in the promoter region of GSDMD gene. Therefore, we hypothesized that NK92 cells pretreatment with DMF or MMF might be inducing the methylation of GSDMD gene. DNA methylation in humans is carried out by a family of enzymes known as DNA methyltransferases (DNMTs). Of which, the DNMT1 is considered to be involved in the maintenance of pre-existing DNA methylation patterns during replication, whereas de novo DNMT activity is largely attributed to DNMT3A or DNMT3B.27 RNA extracted from LPS, DMF or MMF treated NK92 cells was assessed using whole transcriptome expression. The transcriptomic expression for DNMT3A and DNMT3B are shown in Figure 6A. We found the values for DNMT3A and DNMT3B to be 1.6 to 3 fold higher respectively, in DMF-treated NK92 cells as compared to untreated cells. In addition, we found the values for DNMT3A and DNMT3B to be 0.82 to 3 fold change respectively, in MMF-treated NK92 cells when compared to control cells (Figure 6A).
Figure 6.
DMF and MMF induced DNMT-mediated methylation-silencing of GSDMD gene. (A) Whole transcriptome analysis data showed increase in DNMT3A and DNMT3B in DMF- or MMF-treated NK92 cells, compared to control cells. (B) Western blotting data also showed an increase in the expression of DNMT3A and DNMT3B in DMF or MMF treated cells but not in LPS treated cells. (C) DMF and MMF increased the expression of DNMT3A and DNMT3B in cells incubated with LPS. (D) Schematic representation of the presence of CpG Islands in the promoter region of GSDMD gene and repression of expression is observed upon methylation of those CGIs. (E) Data from MSP-PCR showed the methylation levels of GSDMD in cells either untreated (Control), or treated with LPS, or LPS plus DMF or MMF in the presence of IL-2 for 24 h. *P<0.01 compares GSDMD promoter methylation levels in LPS-activated cells vs cells incubated with DMF or MMF. Methylation levels were significantly higher in DMF and MMF treated NK92 cells compared to untreated or LPS treated NK92 cells.
Protein analysis by Western blotting confirmed this up-regulation of DNMTs in DMF- or MMF-treated NK92 cells (Figure 6B). This was also noticed upon LPS-treatment of cells that were pre-treated with DMF or MMF (Figure 6C). After confirming upregulation of DNMTs, we thought to evaluate DNA methylation of GSDMD as these DNMTs are known to induce DNA methylation. Later, this DNMT-mediated hypermethylation of the CpG island in the promoter region of GSDMD could lead to silencing or inhibiting its expression (Figure 6D). In agreement, the MSP-PCR shows that methylation level (ratio of methylated CpG sites/total CpG sites) was increased in NK92 cells pretreated with DMF and MMF compared to untreated cells, as well as cells treated with LPS (Figure 6E), suggesting that DMF or MMF induces hypermethylation resulting in silencing GSDMD, which seems to be mediated by both DNMT3A or DNMT3B.
Discussion
In the present study, we demonstrate several novel findings. The results are the first to show that NK92 cells utilize GSDMD to release the inflammatory cytokine IL-1β. Pyroptosis was detected in NK92 cells by confocal microscopy showing the presence of pyroptotic bodies. This adding to the fact that these cells utilize GSDMD strongly indicates that they may die by pyroptosis. GSDMD has been recently described as the major mediator of pyroptosis.12,13 After activation, this molecule is cleaved into two major components, the inhibitory C-terminal and the pore-forming N-terminal. The amino domain oligomerizes with the plasma membrane phospholipids, particularly phosphatidylinositol PI(4,5)P2 forming pores in these membranes, allowing the exocytosis of pre-formed molecules.
Here, we demonstrate that LPS induced the release of IL-1β from NK92 cells due to the activation of procaspase-1 into an active caspase-1. This is corroborated with the ability of LPS to up-regulate GSDMD in NK92 cells. Further, DMF or MMF impaired LPS-induced IL-1β and the activation of GSDMD. It was previously reported that DMF, and to a lower extent, MMF prevent pyroptosis induced by ATP in THP-1 cells.28 However, our observations are the first to show such activity in NK cells, and the first to demonstrate the anti-death effect of DMF and MMF is related to inhibiting GSDMD gene expression.
Although not more than 20% of NK92 cells express TLR4 the reported receptor for LPS, the appearance of pyroptotic bodies in cells treated with LPS suggests that LPS may induce non-canonical inflammasome activation where excess LPS enter the intracellular environment independent of TLR4, usually contained within vacuoles. Guanylate-binding proteins promote vacuolar lysis, causing entry of LPS into the cytoplasm where it is detected by procaspase-11 initiating the formation of the inflammasome and pyroptosis of the cell.29
Hypermethylation of the promoter region of a gene directly blocks the binding of transcription factors essential for gene expression, thus rendering the loss of expression or gene silencing. Epigenetic mechanisms such as DNA methylation or histone modifications are known to regulate gene expression.30 DNA methylation, one of the most widely studied epigenetic mechanisms, is an addition of a methyl group to the C-5 position of cytosine by a group of enzymes DNA methyltransferase to form 5-methylcytosine. This reaction occurs exclusively on gene promoter regions rich in CG dinucleotide sequence (CpG islands).31 Our search through the UCSC genome browser reveals the presence of CpG island in the promoter region of GSDMD gene. Hence, we hypothesized that treatment of NK92 cells with DMF or MMF treatment might epigenetically regulate the expression of GSDMD. Indeed, our results support the conclusion that DMF or MMF induced the hypermethylation of GSDMD and in turn, this may regulate the release of IL-1β from these cells. Intriguingly, utilizing whole transcriptome analysis, we observed that DMF or MMF upregulated the expression of two enzymes, ie, DNMT3A and DNMAT3B in NK92 cells. These enzymes are known to induce DNA hypermethylation.19,30 Comparison between mRNA and protein expression shows almost similar trend with DNMT3A. However, DNMT3B expression shows some discrepancy between the mRNA and protein expression. Such discrepancy between protein and mRNA is seen in many studies and could be due to post-transcriptional, translational and protein degradation which can affect the regulation of the protein as previously discussed.32
We also sought to demonstrate whether NK92 cells express receptors for DMF or MMF. It has been reported that HCA2 constitutes the receptor for MMF in other cells.25 HCA1 is not known to be expressed on immune cells, but HCA2 and HCA3 have been reported to be expressed in various immune cells such as monocytes and neutrophils but not in lymphocytes.33 In this regard, it was shown that HCA2 mediates the therapeutic effects of DMF or MMF in an experimental autoimmune encephalomyelitis mouse model.25 Hence, our results demonstrating the expression of HCA2 and HCA3 in NK92 cells are novel and could explain the various activities described for DMF or MMF in NK92 cells,17 or human IL-2-activated NK cells.34 Further, the current work is the first to demonstrate that DMF and MMF act as anti-death molecules in NK cells, and the first to show that the anti-death activity of DMF or MMF is related to epigenetic modification of GSDMD in NK92 cells.
In addition to its universal inflammatory function, GSDMD is an important mediator for inducing damages during ischemia-reperfusion brain injury35 and is implicated in the aggressiveness of non-small lung cancer.36 In fact, it was recently suggested that targeting GSDMD is a novel strategy for promoting neuroprotection in multiple sclerosis patients and in mice with experimental autoimmune encephalomyelitis.37 Consequently, molecules such as DMF or MMF that impair its activity, can be used to treat diseases where GSDMD induces damages. Finally, we suggest that because NK cells express many inflammatory chemokines, and respond to these inflammatory molecules,38 it is highly plausible that the exocytosis of these molecules may be due to the activation of GSDMD. Supporting this conclusion is the high percentages of NK92 cells expressing this molecule indicating that it plays pivotal roles in various inflammatory activities mediated by these cells. Although NK cells are equipped with cytolytic, immunoregulatory and tolerance activities,39 pyroptosis could be a process that mediates the immunoregulatory function of NK cells. Although we only examined the release of IL-1β, it is plausible that other cytokines and chemokines might be also secreted from these cells via activation of inflammasome and GSDMD, resulting in pyroptosis. Whether these molecules are involved in NK cell cytotoxicity against tumor cells, has not been examined in this study. Therefore, it is highly important to investigate this issue which may result in finding molecules that could target not only inflammation based disorders, but other diseases such as cancer.
Conclusions
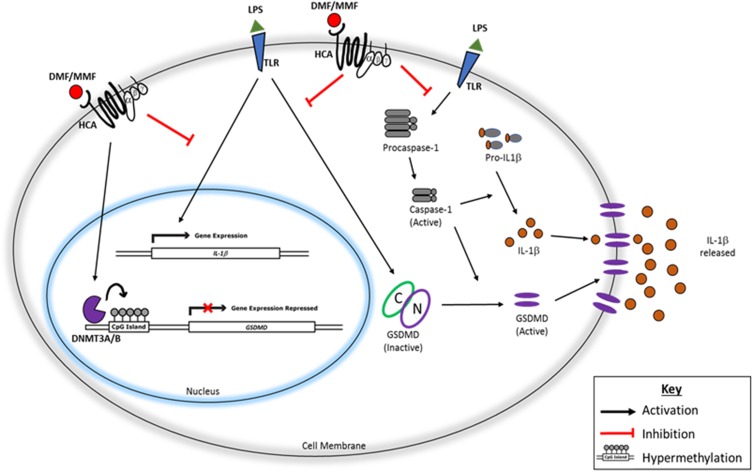
We propose the following mechanism for the drug DMF and for the molecule MMF; after binding their receptors HCA2 or HCA3, these molecules induce the modification of GSDMD gene. GSDMD is activated upon binding inflammatory ligands such as LPS to its respective TLR4, allowing the conversion of procaspase-1 into an activate caspase-1 that cleaves GSDMD into inactive C-terminal and active N-terminal. The latter polymerizes in the plasma membranes, resulting in pyroptotic death of NK cells and the consequent release of IL-1β. These activities are inhibited when DMF or MMF are used due to the hypermethylation of GSDMD gene (Figure 7).
Figure 7.
Schematic representation of the pathway leading to IL-1β release from NK92 and its inhibition by DMF or MMF. After binding TLR (and perhaps others), LPS activates caspase-1 which induces the synthesis of IL-1β. Also, LPS upregulates the mRNA for IL-1β, and stimulates GSDMD which induces pyroptosis in the cell membranes allowing the exocytosis of IL-1β. The activities of GSDMD are inhibited by the drug DMF and by MMF plausibly through hypermethylation of GSDMD gene, resulting in impairing the exocytosis of this cytokine, and preventing the pyroptosis of NK92 cells.
Funding Statement
AAM is supported by the University of Sharjah grant numbers 1701090222-P and 1701090223-P and by Terry Fox Foundation. JSM is supported by the University of Sharjah grant numbers 1801090131-P and 1801090139, and by Al-Jalila foundation grant number AJF2018036. RAH is supported by Al-Jalila grant number AJF201741 and the Sharjah Research Academy grant number MED001.
Abbreviations
ASC, apoptosis-associated speck-like protein containing CARD domain; ATP, adenosine triphosphate; cDNA, complementary deoxyribose nucleic acid; DAPI, 4ʹ,6-diamidino-2-phenylindole; DMF, dimethyl fumarate; DNMTs, DNA methyltransferases; ELISA, enzyme-linked immunosorbent assay; GDNA, genomic deoxyribose nucleic acid; GSDMD, gasdermin-D; HCA, hydroxy-carboxylic acid receptors; IFN-γ, interferon-gamma; IL, interleukin; LPS, lipopolysaccharide; MMF, monomethyl fumarate; MSP-PCR, methylation specific polymerase chain reaction; NK, natural killer; NLRP3, NOD-like receptor pyrin domain containing 3; qPCR, quantitative polymerase chain reaction; TLR, toll-like receptor.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Suck G, Odendahl M, Nowakowska P, et al. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65:485–492. doi: 10.1007/s00262-015-1761-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingemann H, Boissel L, Toneguzzo F. Natural killer cells for immunotherapy - advantages of the NK-92 cell line over blood NK cells. Front Immunol. 2016;7:91. doi: 10.3389/fimmu.2016.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maghazachi AA. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol. 2010;341:37–58. doi: 10.1007/82_2010_20 [DOI] [PubMed] [Google Scholar]
- 4.Sand KL, Knudsen E, Rolin J, Al-Falahi Y. Modulation of natural killer cell cytotoxicity and cytokine release by the drug glatiramer acetate. Cell Mol Life Sci. 2009;66:1446–1456. doi: 10.1007/s00018-009-8726-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolin J, Sand KL, Knudsen E, Maghazachi AA. FTY720 and SEW2871 reverse the inhibitory effect of S1P on natural killer cell mediated lysis of K562 tumor cells and dendritic cells but not on cytokine release. Cancer Immunol Immunother. 2010;59:575–586. doi: 10.1007/s00262-009-0775-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peleg R, Carmi Y, Guttman O, et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. [DOI] [PubMed] [Google Scholar]
- 7.Carmi Y, Dotan S, Rider P, et al. The role of IL-1β in the early tumor cell-induced angiogenenic response. J Immunol. 2013;190:3500–3509. doi: 10.4049/jimmunol.1202769 [DOI] [PubMed] [Google Scholar]
- 8.Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18:192–201. doi: 10.1016/S1470-2045(17)30006-2 [DOI] [PubMed] [Google Scholar]
- 9.Sauter KA, Wood LJ, Wong J, Iordanov M, Magun BE. Doxorubicin and daunorubicin induce processing and release of interleukin-1β through activation of the NLRP3 inflammasome. Cancer Biol Ther. 2011;11:1008–1016. doi: 10.4161/cbt.11.12.15540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, He WT, Hu L, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Muller DJ. Mechanism of membrane pore formation by human gasdermin-D. Embo J. 2018;37:e98321. doi: 10.15252/embj.201798321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monteleone M, Stanley AC, Chen KW, et al. Interleukin-1beta maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Rep. 2018;24:1425–1433. doi: 10.1016/j.celrep.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 13.He WT, Wan H, Hu L, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: a review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;S0003-9861(18)30994–9. [DOI] [PubMed] [Google Scholar]
- 16.Al-Jaderi Z, Maghazachi AA. Utilization of dimethyl fumarate and related molecules for treatment of multiple sclerosis, cancer, and other diseases. Front Immunol. 2016;7:278. doi: 10.3389/fimmu.2016.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elemam NM, Al-Jaderi Z, Hachim MY, Maghazachi AA. HCT-116 colorectal cancer cells secrete chemokines which induce chemoattraction and intracellular calcium mobilization in NK92 cells. Cancer Immunol Immunother. 2019;68:883–895. doi: 10.1007/s00262-019-02319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vego H, Sand KL, Høglund RA, et al. Monomethyl fumarate augments NK cell lysis of tumor cells through degranulation and the upregulation of NKp46 and CD107a. Cell Mol Immunol. 2016;13:57–64. doi: 10.1038/cmi.2014.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muhammad JS, Nanjo S, Ando T, et al. Autophagy impairment by Helicobacter pylori-induced methylation silencing of MAP1LC3Av1 promotes gastric carcinogenesis. Int J Cancer. 2017;140:2272–2283. doi: 10.1002/ijc.30657 [DOI] [PubMed] [Google Scholar]
- 20.Smith TF, Waterman MS. Identification of common molecular subsequences. J Mol Biol. 1981;147:195–197. doi: 10.1016/0022-2836(81)90087-5 [DOI] [PubMed] [Google Scholar]
- 21.Conti P, Dempsey RA, Reale M, et al. Activation of human natural killer cells by lipopolysaccharide and generation of interleukin-1 alpha, beta, tumour necrosis factor and interleukin-6. Effect of IL-1 receptor antagonist. Immunology. 1991;73:450–456. [PMC free article] [PubMed] [Google Scholar]
- 22.Goodier MR, Londei M. Lipopolysaccharide stimulates the proliferation of human CD56+CD3− NK cells: a regulatory role of monocytes and IL-10. J Immunol. 2000;165:139–147. doi: 10.4049/jimmunol.165.1.139 [DOI] [PubMed] [Google Scholar]
- 23.Kanevskiy LM, Telford WG, Sapozhnikov AM, Kovalenko E. Lipopolysaccharide induces IFN-γ production in human NK cells. Front Immunol. 2013;4:11. doi: 10.3389/fimmu.2013.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mian MF, Lauzon NM, Andrews DW, Lichty BD, Ashkar AA. FimH can directly activate human and murine natural killer cells via TLR4. Mol Ther. 2010;18:1379–1388. doi: 10.1038/mt.2010.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Assmann JC, Krenz A, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest. 2014;124:2188–2192. doi: 10.1172/JCI72151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin J, Kostura MJ. Dissociation of IL-1 beta synthesis and secretion in human blood monocytes stimulated with bacterial cell wall products. J Immunol. 1993;151:5574–5585. [PubMed] [Google Scholar]
- 27.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6 [DOI] [PubMed] [Google Scholar]
- 28.Miglio G, Veglia E, Fantozzi R. Fumaric acid esters prevent the NLRP3 inflammasome-mediated and ATP-triggered pyroptosis of differentiated THP-1 cells. Int Immunopharmacol. 2015;28:215–219. doi: 10.1016/j.intimp.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 29.Diamond CE, Khameneh HJ, Brough D, Mortellaro A. Novel perspectives on non-canonical inflammasome activation. Immunotargets Ther. 2015;4:131–141. eCollection 2015. doi: 10.2147/ITT.S57976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhammad JS, Eladl MA, Khoder G. Helicobacter pylori-induced DNA methylation as an epigenetic modulator of gastric cancer: recent outcomes and future direction. Pathogens. 2019;8(1):E23. doi: 10.3390/pathogens8020071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852 [DOI] [PubMed] [Google Scholar]
- 32.Vogel C, Mactotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2013;13:227–232. doi: 10.1038/nrg3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Offermanns S, Colletti SL, Lovenberg TW, Semple G, Wise A, IJ AP. International union of basic and clinical pharmacology. LXXXII: nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B). Pharmacol Rev. 2011;63:269–290. doi: 10.1124/pr.110.003301 [DOI] [PubMed] [Google Scholar]
- 34.Maghazachi AA, Sand KL, Al-Jaderi Z. Glatiramer acetate, dimethyl fumarate, and monomethyl fumarate upregulate the expression of CCR10 on the surface of natural killer cells and enhance their chemotaxis and cytotoxicity. Front Immunol. 2016;7:437. doi: 10.3389/fimmu.2016.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D, Qian J, Zhang P, et al. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J Neurosci Res. 2019;97:645–660. doi: 10.1002/jnr.24385 [DOI] [PubMed] [Google Scholar]
- 36.Gao J, Qiu X, Xi G, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncol Rep. 2018;40:1971–1984. doi: 10.3892/or.2018.6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie BA, Mamik MK, Saito LB, et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci. 2018;115:E6065–E6074. doi: 10.1073/pnas.1722041115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367 [DOI] [PubMed] [Google Scholar]
- 39.Thomas LM. Current perspectives on natural killer cell education and tolerance: emerging roles for inhibitory receptors. Immunotargets Ther. 2015;4:45–53. eCollection 2015. doi: 10.2147/ITT.S61498 [DOI] [PMC free article] [PubMed] [Google Scholar]