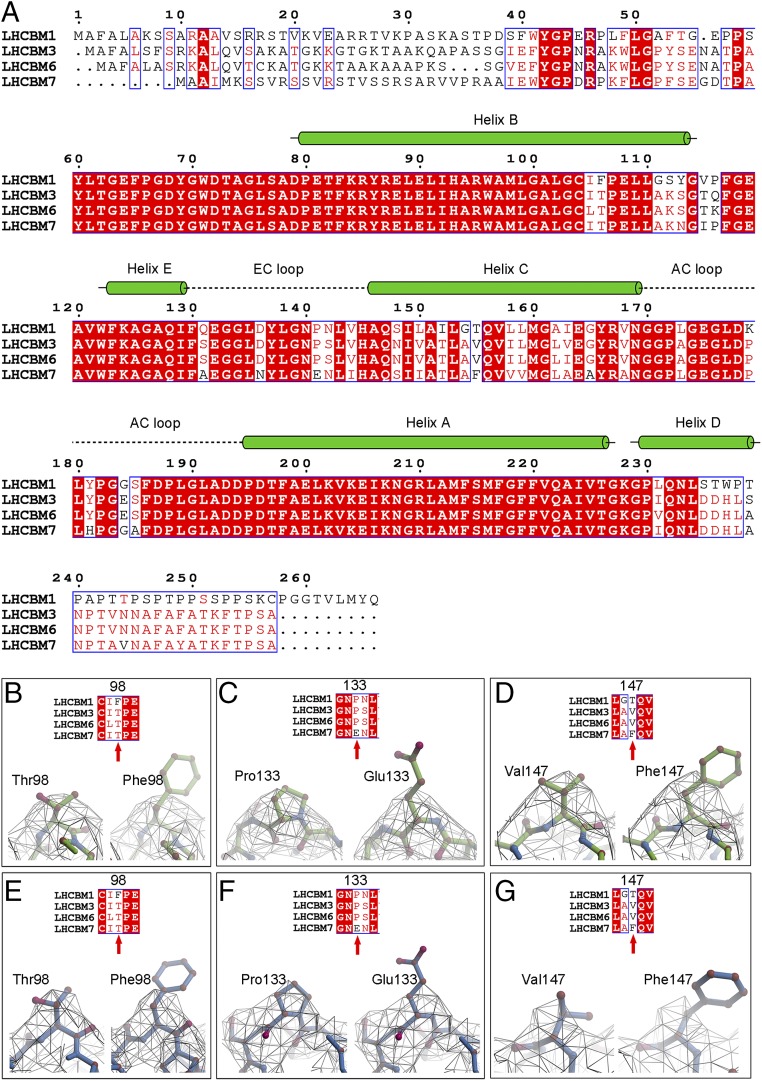
Fig. 3.
Sequence alignment of LHCBM subunits and the assignment of protein subunits in the cryo-EM structure from C. reinhardtii. (A) Sequence alignment of LHCBM1, LHCBM3, LHCBM6, and LHCBM7, which were detected by mass spectrometry from the SDS/PAGE bands of the Cr-PSII–LHCII supercomplex. Information on secondary structures shown above the sequences is taken from the cryo-EM structure of LHCII of the Cr-PSII–LHCII supercomplex obtained here. Helices are represented by green cylinders. Loops are denoted by dashed lines. (B–D) Assignment of the monomer 1 subunit of S-LHCII by comparison of the structural features between amino acids Thr98 and Phe98 (B), Pro133 and Glu133 (C), and Val147 and Phe147 (D). The possibilities of LHCBM1 and LHCBM7 as S-LHCII (monomer 1) in the cryo-EM structure of Cr-PSII–LHCII are excluded on the basis of B–D. (E–G) Assignment of subunit S-LHCII (monomer 3) by comparison of the structural features between amino acids Thr98 and Phe98 (E), Pro133 and Glu133 (F), and Val147 and Phe147 (G). The possibilities of LHCBM1 and LHCBM7 as S-LHCII (monomer 3) in the cryo-EM structure of Cr-PSII–LHCII are excluded on the basis of E, F and G. Thus, either LHCBM3 or LHCBM6 can be assigned to S-LHCII (monomer 1) or S-LHCII (monomer 3).