Significance
Laminar resolution, functional MRI (lfMRI) is a noninvasive technique with the potential to distinguish top-down and bottom-up signal contributions on the basis of laminar specific interactions between distal regions. Hitherto, lfMRI could not be demonstrated for either whole-brain distributed networks or for complex cognitive tasks. We show that lfMRI can reveal whole-brain directed networks during word reading. We identify distinct, language-critical regions based on their association with the top-down signal stream and establish lfMRI for the noninvasive assessment of directed connectivity during task performance.
Keywords: laminar fMRI, systems neuroscience, BOLD biophysics, directed connectivity, language
Abstract
Interactions between top-down and bottom-up information streams are integral to brain function but challenging to measure noninvasively. Laminar resolution, functional MRI (lfMRI) is sensitive to depth-dependent properties of the blood oxygen level-dependent (BOLD) response, which can be potentially related to top-down and bottom-up signal contributions. In this work, we used lfMRI to dissociate the top-down and bottom-up signal contributions to the left occipitotemporal sulcus (LOTS) during word reading. We further demonstrate that laminar resolution measurements could be used to identify condition-specific distributed networks on the basis of whole-brain connectivity patterns specific to the depth-dependent BOLD signal. The networks corresponded to top-down and bottom-up signal pathways targeting the LOTS during word reading. We show that reading increased the top-down BOLD signal observed in the deep layers of the LOTS and that this signal uniquely related to the BOLD response in other language-critical regions. These results demonstrate that lfMRI can reveal important patterns of activation that are obscured at standard resolution. In addition to differences in activation strength as a function of depth, we also show meaningful differences in the interaction between signals originating from different depths both within a region and with the rest of the brain. We thus show that lfMRI allows the noninvasive measurement of directed interaction between brain regions and is capable of resolving different connectivity patterns at submillimeter resolution, something previously considered to be exclusively in the domain of invasive recordings.
Top-down and bottom-up information streams are integral to brain function but notoriously difficult to measure noninvasively. In this work, we infer directed interaction between language-relevant regions by using functional MRI with laminar resolution (lfMRI).
Language processing is challenging to understand, as it draws on regions throughout the brain which interact in complex, dynamic configurations. Changes in blood oxygen level-dependent (BOLD) amplitude in specific regions have been observed in response to linguistic demands, such as lexical retrieval (posterior left middle temporal gyrus; pLMTG) or the process of combining words into phrases (left inferior frontal gyrus, discussed in ref. 1). During reading, activation in a portion of left occipitotemporal cortex is commonly observed, in addition to activation of language-critical regions such as pLMTG (see refs. 2 and 3). Our understanding of such networks would be greatly enhanced if we could elucidate the top-down and bottom-up influences on constituent regions. We show in this work that lfMRI can circumvent current methodological limitations which preclude direct, noninvasive measurements of directed interaction.
Anatomists have long been aware of the laminar structure of mammalian neocortex (4–7) and have considered its functional implications (8). The advent of retrograde tracers (9) and, more recently, of viral tracing methods has since expanded our understanding of laminar circuits (10, 11). A key property of laminar circuits is that extrinsic connections to a region tend to target specific layers depending on the hierarchical relationship between the regions. A generalized characterization of this pattern holds that top-down connections preferentially target the deep and superficial layers, whereas bottom-up connections preferentially target the middle layer (8, 12, 13).
With advances in high-field MRI, it has become feasible to explore laminar specific functional imaging. A substantial body of work assessing the laminar sensitivity of neurovascular mechanisms indicates that hemodynamic-based measures can be well localized to the site of activation (14–17, reviewed in ref. 18). This suggests that lfMRI is capable of distinguishing signal driven by neuronal activity in different cortical layers corresponding to hierarchically distinct information streams. Research on this topic has led to reports of task-modulated effects at depths associated with the top-down and bottom-up termination sites of mostly sensory (19–23) and motor cortices (24), and at least 1 laminar resolution experiment has been performed in prefrontal cortex (25). In addition, the BOLD signal has been linked to oscillatory activity in different frequency ranges as a function of cortical depth (26). Optical imaging and electrophysiological research in mice has also shown evidence for layer-specific connectivity across motor and visual cortex unique to delta (1 to 4 Hz) and infraslow (<0.1 Hz) frequency ranges (27).
While these previous studies dissociated top-down and bottom-up signal within a region, we demonstrate that these signal streams preserve source information and can be used to identify directed networks.
In this work, we extracted depth-dependent time courses from submillimeter BOLD measurements. We show that reading words compared to pseudowords increased the top-down BOLD signal observed in the deep layers of the left occipitotemporal sulcus (LOTS). The depth-dependent responses indicated that different cognitive processes occurred at distinct depths and would not have been observed at standard resolutions. The depth-dependent measurements were then used to identify distinct distributed networks corresponding to top-down and bottom-up signal pathways which targeted the LOTS during word reading. The depth-dependent signals demonstrated unique connectivity patterns with other regions, thereby establishing the directionality of interaction within the reading network. In addition to the discovery that directed connectivity can be explicitly measured through lfMRI, this work provides direct evidence that top-down signal to the LOTS is involved in word reading.
Results
Top-Down Condition-Specific Effects in LOTS.
We manipulated the top-down signal directed toward LOTS by visually presenting words and pseudowords in Dutch (explained in Fig. 1). Pseudowords are nonsense words that are orthographically and phonologically legal. The paradigm contrasted 2 potential means of eliciting a top-down response to the LOTS. In the first, as visual representations of words are related to information unavailable from bottom-up sensory information, it might be possible to observe a relative increase in top-down signal during word reading owing to the linguistic information that could be retrieved from memory (28, 29). In the second, pseudoword reading is known to be more effortful and to evoke a larger BOLD response in the LOTS. A larger top-down contribution during pseudoword reading might therefore be expected to contribute to the enhanced BOLD response in the region. The contrast effect, that is, words against pseudowords, was expected to target histological layers VI/V or III/II/I. As discussed, these layers are known to contain extrinsic feedback targets and were subsumed within the deep (VI, V) or superficial (III/II/I) bins in our layering scheme (Fig. 2).
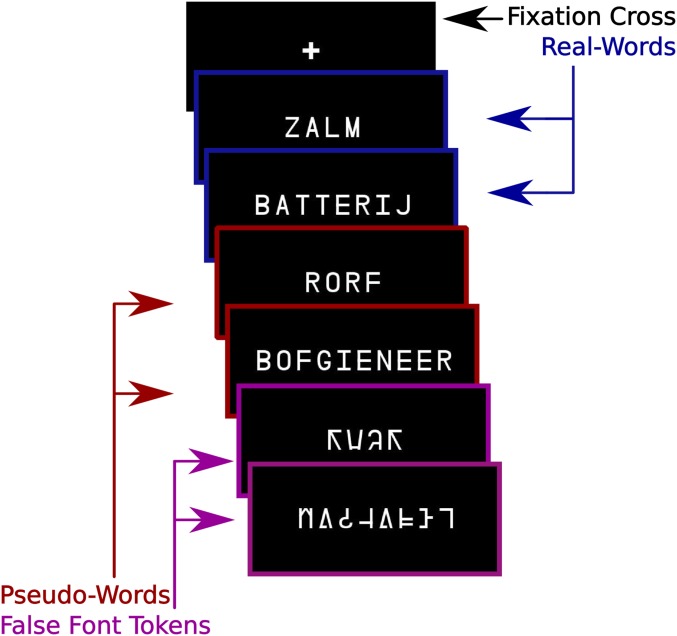
Fig. 1.
Sample stimuli from each task condition. Word stimuli are in Dutch. English translations of the Dutch word stimuli examples are “salmon” and “battery.” Stimuli were presented in 5-item miniblocks with the items from the same condition presented in each block.
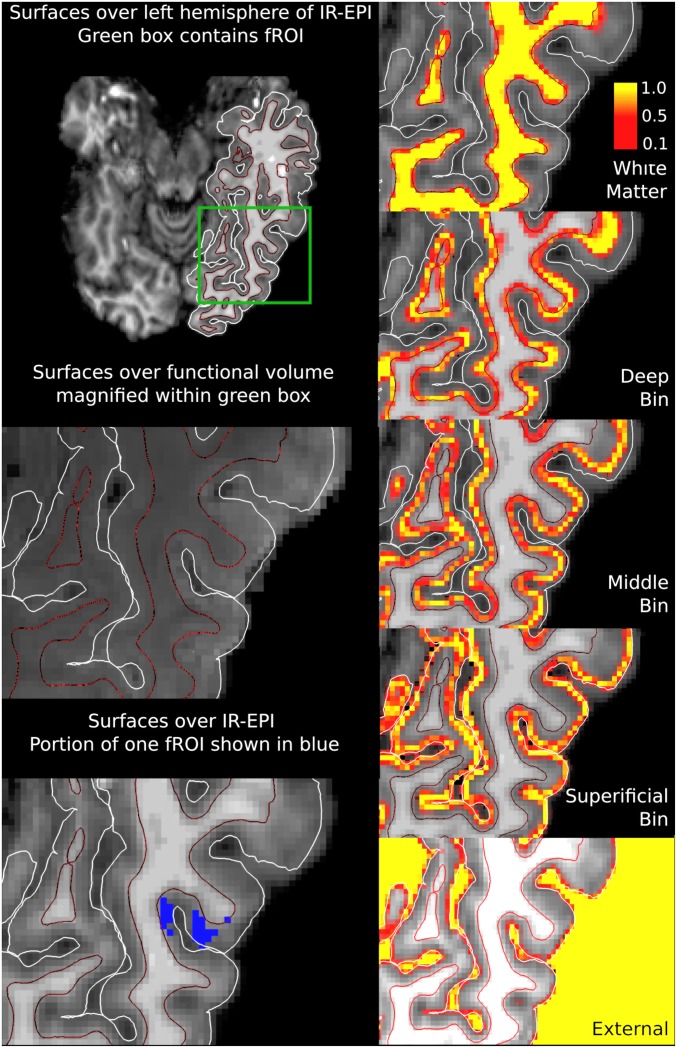
Fig. 2.
Surfaces and volumetric layering in 1 subject. (Left) White matter (shown in red/black) and pial (shown in white) surfaces are shown overlaid on anatomical and T2*- weighted images. Slice through fROI is shown in blue. Magnification within the green box highlights fROI location. (Right) Equivolume depth bins shown in volume space. Each voxel was assigned 5 values between 0 and 1 according to the proportion of its volume in each of the depth bins. A value of 1 (yellow) indicates that the voxel is fully contained within a given depth bin, 0 (black) that it is not within the depth bin. Intermediate values indicate the fractional volume within a given bin.
Submillimeter gradient echo (GE) BOLD 3D echo-planar imaging (30) data were acquired and analyzed in 22 native Dutch participants during a single word reading paradigm. Participants were instructed to silently read each item presented on the screen. Participants were also presented with items printed in a “false font,” an artificial script designed to conserve the low-level features (e.g., angular properties) of the common Latin script. Characters of a false font are letterlike but visually distinct from characters in orthographies known to participants (Fig. 1). After some miniblocks of trials, participants were asked to indicate via button box if the miniblock contained existing words. (2). A detailed visualization of the paradigm can be found in SI Appendix.
By contrasting the BOLD response of the false-font items against the words/pseudowords, it was possible to identify a region that responded preferentially to the orthographic and lexical features of a stimulus rather than to its low-level visual properties. This functionally defined region in the LOTS is sometimes referred to as the “visual word form area” (31). The network properties of this region are of interest, as it is thought that top-down signal directed toward this region may facilitate the process of associating visual information—the sensory information related to the visual representation of the words—with linguistic information (29). The depth-dependent measures reported here were taken from this functionally defined region of interest (fROI).
To analyze the depth-dependent BOLD signal, the gray matter volume of the fROI in each subject was divided into 3 equivolume depth bins following Waehnert et al. (32), roughly containing the deep, middle, and superficial layers, respectively. We restricted our predictions, however, to deeper cortex given that previous work with GE BOLD has discovered top-down effects in the deep bin (22, 23). BOLD activity observed in the deep bin was thus taken to best express activity arising from top-down sources, whereas the middle bin was considered to express bottom-up related activity. The depth-dependent voxel contributions within the fROI were modeled with a spatial general linear model (GLM) (33). A temporal GLM was then fitted to the depth-dependent time courses that had been computed for each bin using the spatial GLM.
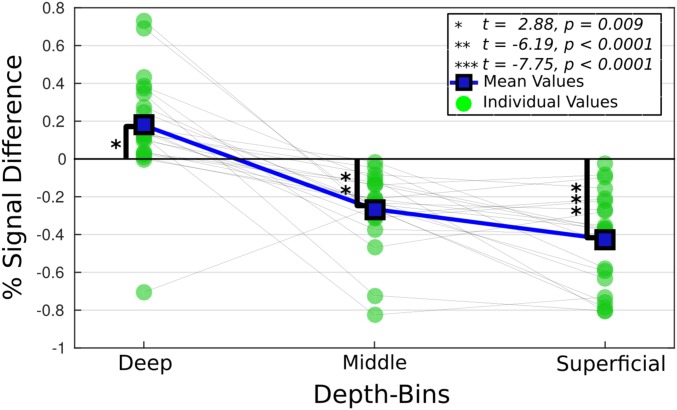
A 3-way ANOVA was performed to assess the main effects of depth and condition (words, pseudowords) as well as their interaction. Participants were modeled as a random factor. Significant main effects were found for both depth [F(2,105) = 84.37, P < 0.001] and condition [F(1,105) = 10.98, P = 0.0013]. The depth condition interaction was also significant [F(2,105) = 12.24, P < 0.001]. The nature of this interaction was further tested by t statistics on the word and pseudoword response contrast at each depth bin (Fig. 3).
Fig. 3.
The difference in percent signal change between words and pseudowords is shown by depth for all participants. Significance bars relate to t statistics computed on the difference from 0 (n = 22).
The depth-dependent t statistics were reduced in the middle and superficial bins for words relative to pseudowords. T statistics in the deep bin were, however, greater for the word condition (Fig. 3). The greater t statistic in the deep bin is evidence for elevated top-down related signal in the LOTS for word compared to pseudoword reading. The concurrent reduction of the t statistics within the middle and superficial bins suggests that the increased top-down signal acted to facilitate word reading and was related to the globally attenuated BOLD response throughout the LOTS, as proposed in ref. 29.
While the relative top-down information content was varied through the word/pseudoword manipulation, the experiment did not include a bottom-up manipulation. The bottom-up information content was therefore held constant across conditions, and the middle bin effect was unlikely to be the result of differential bottom-up stimulation.
Within-Region Condition-Specific Bin-to-Bin Interaction Effects.
Generalized psychophysiological interaction analysis (gPPI) is commonly performed on fMRI data to fit terms in a GLM framework which model the interaction between task conditions (i.e., words and pseudowords in our experiment) and brain regions (34). The goal is typically to assess the interaction between brain regions in the context of an experimental task. One interpretation of the gPPI analysis is that the strength of the interaction relates to the tendency of the seed region to enhance the response of the target region to a task condition. We used this technique to assess the condition-specific interactions between the depth bins within the LOTS.
The gPPI model contained all terms used in the first-level model with the addition of terms modeling the interaction between the time courses of the individual depth bins and the task conditions. These were fitted for each participant, and group-level t statistics were then computed from the fitted interaction term parameters. The t statistics represent the pairwise effect of each bin on every other bin and contrasted the word and pseudoword conditions to determine if interbin signal modulation contributed to the lexicality effect shown in Fig. 3.
Activity in the deep bin was shown to predict decreased activity in the middle bin during word reading compared to pseudoword reading. This is observed as a negative interaction effect during word reading, indicating that activation in the deep bin predicted a reduced response in the middle bin during word compared to pseudoword reading, or equivalently an increased response for pseudowords compared to words. Taken together with the increased deep bin and reduced middle bin t statistics observed during word reading in Fig. 3, the gPPI results suggest that increased top-down signal during word reading had a suppressive effect on the middle bin, and perhaps on the global signal in the region (Table 1).
Table 1.
Bin-to-bin intraregional gPPI
| Seed | Target | t value | P value |
| Deep | Middle | −3.429 | 0.003* |
| Deep | Superficial | −2.194 | 0.04 |
| Middle | Deep | −0.9677 | 0.35 |
| Middle | Superficial | −0.3773 | 0.71 |
| Superficial | Deep | −1.655 | 0.11 |
| Superficial | Middle | −0.8662 | 0.34 |
Table shows t statistics for contrast of the word compared to pseudoword conditions. Seed regions were modeled in the gPPI analysis to explain variance in target regions. The deep bin shows reduced interaction with the middle bin during word reading. *P≤0:01, n = 22.
Depth-Dependent Whole-Brain Interactions.
A second gPPI analysis was performed to examine the effect of the LOTS depth bins on the BOLD response throughout the entire brain. As commonly applied, gPPI analysis is not suited to the study of directed interactions between brain regions. By performing gPPI on depth-dependent data, it is, however, possible to leverage knowledge of interregional laminar connectivity patterns to obtain directional information. Thus, gPPI on depth-dependent data can be regarded as a directed measure, capable of capturing the hierarchical relationship between distinct regions in a distributed network.
The whole-brain gPPI model included the interaction terms for the depth bins (deep and middle) and the task conditions (words and pseudowords). These were then fitted to spatially normalized data in each participant to identify condition-specific, depth-dependent networks at the group level. No spatial normalization was applied to the depth bins. The depth bin time courses (deep and middle) and their interaction (deep × middle) were also modeled and used to assess task-independent connectivity for each depth bin. The interaction between the seeds was included to model intraregional interactions of one bin on another. GPPI targets predicted by the deep bin terms were interpreted as being components of a top-down network related through LOTS. Those predicted by middle bin terms were considered components of a bottom-up network.
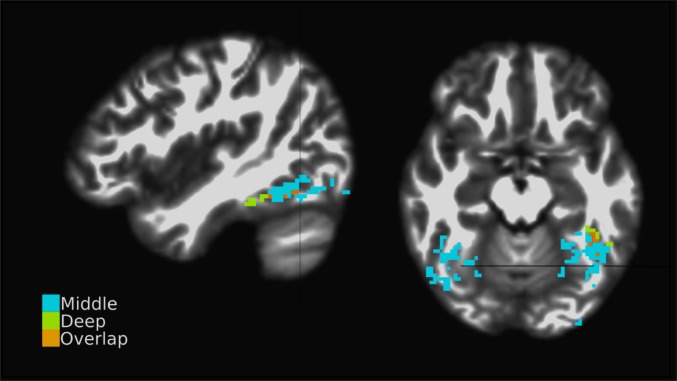
Strikingly, the deep and middle bins did not interact with similar brain regions. In the task-independent analysis, we calculated t statistics for the deep and middle bin time-course estimates to obtain a pseudo resting state connectivity measure. Notably, the middle bin time course experienced strong interactions with bilateral regions posterior to the LOTS, indicative of a feed-forward network including early visual regions. The deep bin interacted with left lateralized gPPI targets anterior to the LOTS, an expected source of top-down afferents to the region. The difference in regional specificity between the 2 bins is noteworthy. The deep bin interacted with regions within a small volume. By comparison, middle bin interactions were distributed throughout a large bilateral volume (Fig. 4).
Fig. 4.
Task-independent connectivity. Colors indicate clusters targeted by the middle bin, deep bin, or both. Cross-hairs indicate the center of mass of the Montreal Neurological Institute (MNI) normalized group average fROI (MNI coordinates: −43.8, −62.0, −15.7). The left hemisphere is shown on the right of the axial image, and the sagittal aspect shows the left hemisphere. , , n = 21.
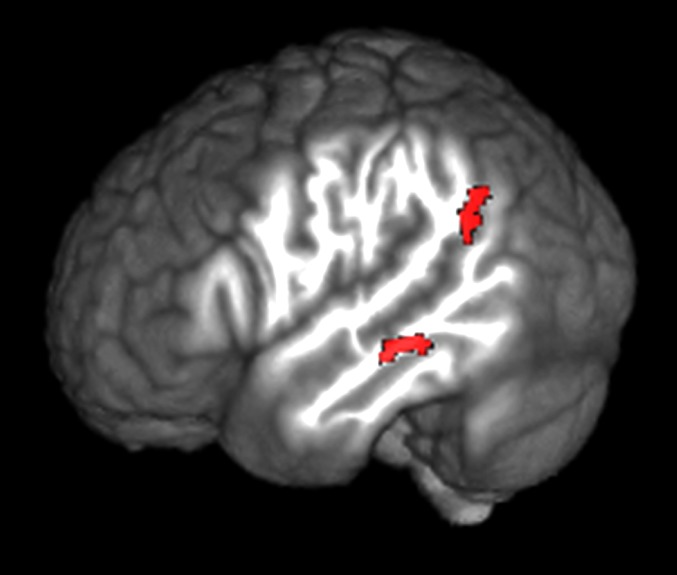
The depth-dependent interaction terms of the task-dependent analysis identified a top-down network sensitive to the lexicality contrast. The identified network included language-critical regions in left temporal cortex and responded preferentially to word reading over pseudoword reading. When corrected for multiple comparisons (Puncorr = 0.001, = 0.05), the deep bin exclusively targeted left middle temporal gyrus (lMTG) and pLMTG, regions commonly associated with lexical retrieval and semantic processing (1, 2, 35) (Fig. 6). The middle bin showed reduced interaction generally, with no clusters surviving correction. A summary of all results is visualized in Fig. 5.
Fig. 6.
Words against pseudowords gPPI for the deep bin. Shown in red: the deep bin preferentially targets left lateralized, language-critical regions during word reading. , , n = 21.
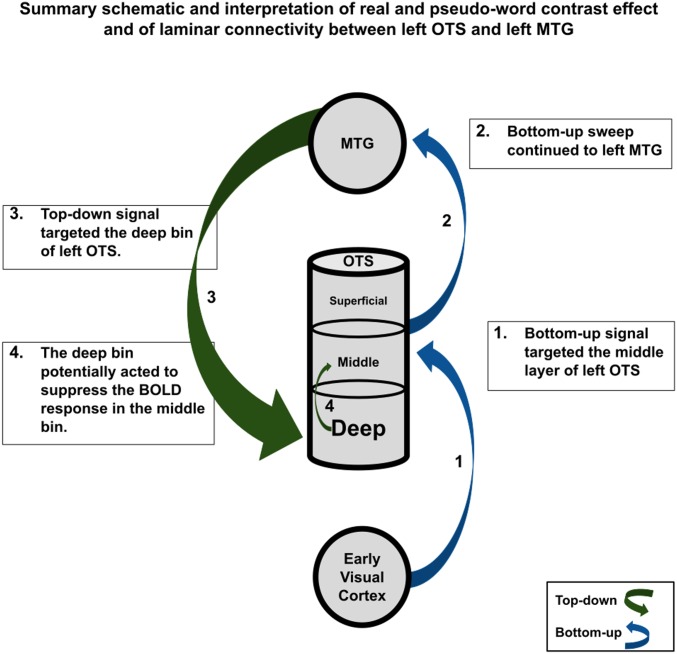
Fig. 5.
Participants read words and pseudowords, and we examined this contrast. The deep bin responded preferentially to words, whereas the middle and superficial bins responded preferentially to pseudowords (contrast effect indicated by text size within the OTS node). Connectivity was observed to be stronger during word reading between the LOTS and LMTG (indicated by arrow thickness) and related uniquely to the signal observed in the deep bin of the LOTS.
Discussion
The sensitivity of adjacent depth bins to distinct regions in distributed networks is our most important finding. The unique connectivity profiles associated with each depth bin empirically demonstrate the ability of lfMRI to noninvasively identify directed networks. We attribute this outcome to previously unknown characteristics of depth-dependent GE BOLD in combination with gPPI analysis. The findings are direct evidence of top-down interaction between language-critical regions in the temporal cortex and the LOTS, relative to the LOTS. Information flow between the temporal and occipital regions is therefore best characterized in terms of top-down rather than bottom-up signaling, a finding which provides insight into the functional role of these regions during word reading. Beyond the unique contribution to the neurobiology of language, the ability to measure directed connectivity has far-reaching consequences for expanding our knowledge of brain networks and the processes they implement.
Depth-Dependent Response to Conditions in LOTS.
The reading paradigm used in this work manipulated the top-down information load while holding the sensory input constant across task conditions. The comparatively greater amount of top-down information during word reading resulted in increased top-down signal and elevated BOLD response in the deep layers of the LOTS. The depth-dependent t statistics calculated on the contrast of the word and pseudoword task condition parameters (Fig. 3) revealed a relative increase in activation in the deep bin during word reading, but a decrease in the middle and superficial bins. The overall response observed when integrating over the 3 depth bins was reduced, or suppressed, for word reading compared to pseudoword reading, indicating that the global response did not represent the contrast effect in each bin. This result is evidence that distinct processes occurred within discrete depth compartments during a single condition, and that word reading led to signal increases exclusively in the deep bin. It also illustrates the sensitivity of lfMRI to this nuance which would have been missed by conventional fMRI.
It is not surprising that word reading resulted in a globally suppressed BOLD response in the LOTS given the well-known findings from reading experiments (2), but the degree of independence between the deep and other bins is striking. In the presence of global signal reduction, the deep bin expressed increased activation. The relationship between the signal observed in the depth bins to the global signal is interesting and could be uniquely explored in this experiment. This was addressed in the intraregional gPPI analysis and is discussed in the following section.
It is generally agreed that synaptic terminations in layer IV strongly excite their targets and drive spike-rate increases throughout the layers of the target region (36). It was hitherto unknown, however, whether comparatively subtle changes in top-down related responses would be detectable in the BOLD signal in the presence of bottom-up responses. The robust deep bin effect during the word condition indicates that top-down manipulations could indeed be detected without experimentally altering the bottom-up signal, at least with respect to the deep bin. Although it is relatively simple to experimentally reduce bottom-up stimulation in low-level sensory paradigms, the requirement to do so would pose a challenge to the research of higher-order cognitive phenomena and limit the potential of lfMRI. This finding suggests that lfMRI might be useful to investigate brain networks whose top-down and bottom-up components are presently unclear, and in cases where it is not practical to manipulate the bottom-up signal.
Bin-to-Bin gPPI.
Results from the intraregional gPPI analysis are evidence that the distinct responses within discrete depth compartments arose in part through interactive signal modulation between depth bins.
Observations in the middle bin related not only to the condition-specific response but were also the result of stimulus-specific interaction with the deep bin. The gPPI analysis revealed a diminished interaction between the deep and middle bins during word compared to pseudoword reading, which we interpret as suppression of the middle bin driven by the deep bin (Table 1). In the depth-dependent analysis summarized in Fig. 3, the deep bin expressed a higher t statistic during word compared to pseudoword reading, despite the lower t statistics from the middle and superficial bins. We see these results as evidence of top-down connectivity during word reading, but they also suggest that signal in the deep bin acted to suppress signal in the middle bin. When the reduced interaction (Table 1) is considered together with the depth-dependent t statistics, it is most plausible that the suppression of the global signal during word reading is attributable to the increased deep bin effect.
It should be mentioned that a clear asymmetry is observed when reversing seed/target pairs. The deep and middle bin only interacted such that the deep bin influenced the middle bin, and the middle bin was not observed to influence the deep bin. Given the predictive nature of gPPI there is no reason to expect that reversing the seed/target pairs should return similar results, as would be expected from a correlational measure. As discussed in the seminal PPI paper (37), activity in one brain region can be predicted by activity in another on the basis of the contribution from the second region to the first. The PPI method does not imply symmetrical contribution between 2 interacting brain regions.
In summary, the intraregional results provide evidence in favor of top-down facilitation during word compared to pseudoword reading (29) and suggest that activity in the deep layers can act to suppress activation in the middle layers. This mechanistic, interlaminar description of suppression suggests new possibilities in the use of functional measures to investigate intrinsic connectivity at the mesoarchitectural scale.
Task-Independent Whole-Brain Connectivity.
The connectivity patterns observed in the task-independent gPPI results (Fig. 4) are in agreement with the expected organization of bottom-up and top-down efferent signal streams in the visual processing hierarchy (9). Relative to a single region, the bottom-up signal is by necessity uninterrupted throughout the processing hierarchy. The top-down stream by comparison does not have these constraints and may be restricted to fewer regions. The larger volume of the middle bin gPPI targets and the smaller volume of the deep bin gPPI targets are consistent with these constraints. The anterior/posterior spatial distribution of the targets is consistent with the top-down/bottom-up signal streams in occipital cortex. Bottom-up signal originates in lower regions relative to efferent targets, primarily in visual cortex, for the LOTS. Top-down signal originates in higher regions, often anterior to target populations. Finally, the bilateral middle bin interactions are consistent with the LOTS receiving input from bilateral sources (38).
Condition-Dependent Connectivity.
The increased connectivity to the pLMTG and lMTG during word reading was only to the deep bin, which we interpret as evidence of a top-down reading network relative to LOTS (Fig. 6). No middle bin interactions survived correction. It is remarkable that the different depth bins expressed this degree of specificity and that they could be used to investigate directed interactions instantiating high-level cognitive phenomena. The deep bin condition-specific interaction is direct evidence that the temporal cortex regions in the reading network relate to the LOTS through top-down rather than bottom-up signal. It is therefore unlikely that the LOTS acts as a node in a forward-directed reading network (31), but rather that word reading is an interactive process whereby top-down signal from temporal cortex to LOTS augments the integration of visual information with linguistic knowledge (29).
It was not previously known that the commonly used GE BOLD contrast would be capable of resolving spatially adjacent BOLD responses with sufficient accuracy to interrogate the depth-dependent connectivity of distributed networks, or to observe interactions among bins within a region. We attribute these findings to a combination of factors. First among these is the fact that the gPPI analysis regressed out the main effects of the task conditions which best captured variance shared across multiple bins.
Furthermore, previous work has demonstrated that the GE BOLD response has a peak in the layer in which it originates and a flat tail of far lower intensity up to the pial surface (39). Variance unique to a given layer will therefore be weaker outside the layer and likely below the threshold for detectability. It follows that some amount of signal contamination will occur from deeper to more superficial layers, but that it should be best represented in the main effects of the task conditions. We argue that the gPPI analysis accounted for downstream signal contamination by regressing out the main effects of the task conditions. The interaction terms could then localize unique variance to the correct depth bins. While it was not the focus of this study, these results further support the notion that the neurovascular response is highly linear and spatially tightly coupled, as previously reported for optical imaging techniques (40).
This work introduces possibilities for noninvasively exploring the interaction between brain regions at a spatial scale previously limited to invasive recordings. The benefits of directional connectivity measurements during a task were demonstrated here in the reading network but are potentially applicable to the study of interregional systems throughout the brain. By targeting the reading network, we demonstrated use of lfMRI to observe connectivity across multiple nonsensory brain regions during the execution of a uniquely human capacity. It seems likely then that the fundamental approach taken in this paper generalizes to different brain regions and to different topics of inquiry. Distributed networks are known to be critical for brain function, and expanding our study of these systems will ultimately improve our understanding of how higher-level functions are instantiated.
Methods and Materials
Twenty-four native Dutch subjects performed a word reading task which presented single words, pseudowords, and false-font items while lying in a 7-T MRI scanner. Two subjects were ultimately excluded, leaving 22 for analysis. Subjects had normal or corrected-to-normal vision and were screened for reading impairment. Informed consent for all experimental procedures was obtained in accordance with procedures of ethical approval of the Donders Center for Cognitive Neuroimaging and the The Erwin L. Hahn Institute.
The experiment included 3 conditions (words, pseudowords, and false-font items) with 240 items for each condition. A length manipulation was initially planned but was shown to be ineffective on the basis of a pilot experiment. This manipulation was excluded from analysis. Detailed information pertaining to the experiment design can be found in SI Appendix.
The fROI was identified in each subject using an anatomically restricted contrast of the 3 conditions. A region was considered for inclusion if it fell within the extent of the LOTS and responded preferentially to words and pseudowords compared to false-font items. The full procedure is described in SI Appendix.
Three equivolume bins were calculated over the fROI in each subject following Waehnert et al. (32). A spatial GLM was then used to extract the depth-dependent time courses from the fROI in each subject (33). The depth-dependent responses to the experimental conditions were assessed in a group-level, 3-way ANOVA. A subsequent 2-tailed, paired t test then examined the responses to the word and pseudoword contrast. gPPI was used to measure the group-level connectivity between depth compartments within the fROI as well as the directed connectivity between the depth compartments and the rest of the brain. See SI Appendix for a complete description of the analysis methods used in this experiment.
Supplementary Material
Acknowledgments
We thank those who contributed to this work, especially research assistants and technical staff. Support was provided by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO), Language in Interaction Gravitation Grant (024.001.006).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907858116/-/DCSupplemental.
References
- 1.Hagoort P., MUC (memory, unification, control) and beyond. Front. Psychol. 4, 416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price C. J., A review and synthesis of the first 20 years of pet and fmri studies of heard speech, spoken language and reading. Neuroimage 62, 816–847 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreiras M., Blair C. A., Perea M., Frost R., The what, when, where, and how of visual word recognition. Trends Cogn. Sci. 18, 90–98 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Campbell A. W., Histological Studies on the Localisation of Cerebral Function (Cambridge University Press, 1905). [Google Scholar]
- 5.Brodmann K., Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien Dargestellt auf Grund des Zellenbaues (Barth, 1909). [Google Scholar]
- 6.Constantin Freiherr von Economo , Koskinas G. N., Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen (Springer, 1925). [Google Scholar]
- 7.Cajal R. Y., Histologie du syst nerveux de l’homme des vert (Maloine, Paris, 1911), vol. 2. [Google Scholar]
- 8.Douglas R. J., Martin K. A., Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 27, 419–451 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Rockland K. S., Pandya D. N., Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179, 3–20 (1979). [DOI] [PubMed] [Google Scholar]
- 10.Barbas H., General cortical and special prefrontal connections: Principles from structure to function. Annu. Rev. Neurosci. 38, 269–289 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Rockland K. S., What do we know about laminar connectivity? Neuroimage 197, 772–784 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Felleman D. J., Van Essen D., Distributed hierarchical processing in the primate cerebral cortex. Cerebr. Cortex 1, 1–47 (1991). [DOI] [PubMed] [Google Scholar]
- 13.Douglas R. J., Martin K. A. C., Mapping the matrix: The ways of neocortex. Neuron 56, 226–238 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Duong T. Q., Silva A. C., Lee S.-P., Kim S.-G., Functional MRI of calcium-dependent synaptic activity: Cross correlation with CBF and BOLD measurements. Magn. Reson. Med. 43, 383–392 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Jin T., Kim S. G., Cortical layer-dependent dynamic blood oxygenation, cerebral blood flow and cerebral blood volume responses during visual stimulation. Neuroimage 43, 1–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goense J. B. M., Logothetis N. K., Laminar specificity in monkey V1 using high-resolution SE-fMRI. Magn. Reson. Imag. 24, 381–392 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Goense J., Merkle H., Logothetis N. K., High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative bold responses. Neuron 76, 629–639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norris D. G., Polimeni J. R., Laminar (f)MRI: A short history and future prospects. Neuroimage 197, 643–649 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Koopmans P. J., Barth M., Norris D. G., Layer-specific bold activation in human V1. Hum. Brain Mapp. 31, 1297–1304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muckli L., et al. , Contextual feedback to superficial layers of V1. Curr. Biol. 25, 2690–2695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Martino F., et al. , Frequency preference and attention effects across cortical depths in the human primary auditory cortex. Proc. Natl. Acad. Sci. U.S.A. 112, 16036–16041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kok P., Bains L. J., van Mourik T., Norris D. G., de Lange F. P., Selective activation of the deep layers of the human primary visual cortex by top-down feedback. Curr. Biol. 26, 371–316 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Lawrence S. J. D., et al. , Laminar organization of working memory signals in human visual cortex. Curr. Biol. 28, 3435–3440.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Huber L., et al. , High-resolution cbv-fmri allows mapping of laminar activity and connectivity of cortical input and output in human m1. Neuron 96, 1253–1263.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn E. S., Huber L., Jangraw D. C., Bandettini P. A., Layer-dependent activity in human prefrontal cortex during working memory. bioRxiv:10.1101/425249 (24 September 2018). [DOI] [PMC free article] [PubMed]
- 26.Scheeringa R., Koopmans P. J., van Mourik T., Jensen O., Norris D. G., The relationship between oscillatory eeg activity and the laminar-specific bold signal. Proc. Natl. Acad. Sci. U.S.A. 113, 6761–6766 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra A., et al. , Spontaneous infra-slow brain activity has unique spatiotemporal dynamics and laminar structure. Neuron 98, 297–305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray J., “What’s in the lexicon? ” in Storage and Computation in the Language Faculty, Nooteboom S. G., Ed. (Springer, 2002) pp. 23–58. [Google Scholar]
- 29.Price C. J., Devlin J. T., The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn. Sci. 15, 246–253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poser B. A., Koopmans P. J., Witzel T., Wald L. L., Barth M., Three dimensional echo-planar imaging at 7 tesla. Neuroimage 51, 261–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dehaene S., Le Clec’H G., Poline J. B., Le Bihan D., Cohen L., The visual word form area: A prelexical representation of visual words in the fusiform gyrus. Neuroreport 13, 321–325 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Waehnert M. D., et al. , Anatomically motivated modeling of cortical laminae. NeuroImage 93, 210–220 (2014). [DOI] [PubMed] [Google Scholar]
- 33.van Mourik T., van der Eerden J. P. J. M., Bazin P.-L., Norris D. G., Laminar signal extraction over extended cortical areas by means of a spatial GLM. PLoS One 14, e0212493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaren D. G., Ries M. L., Xu G., Johnson S. C., A generalized form of context-dependent psychophysiological interactions (gppi): A comparison to standard approaches. NeuroImage 61, 1277–1286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snijders T. M., et al. , Retrieval and unification of syntactic structure in sentence comprehension: An fMRI study using word-category ambiguity. Cerebr. Cortex 19, 1493–1503 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Bastos A. M., et al. , Canonical microcircuits for predictive coding. Neuron 76, 695–711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friston K. J., et al. , Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6, 218–229 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Chu R. K., Meltzer J. A., Interhemispheric connectivity during lateralized lexical decision. Hum. Brain Mapp. 40, 818–832 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markuerkiaga I., Barth M., Norris D. G., A cortical vascular model for examining the specificity of the laminar bold signal. Neuroimage 132, 491–498 (2016). [DOI] [PubMed] [Google Scholar]
- 40.O’Herron P., et al. , Neural correlates of single-vessel haemodynamic responses in vivo. Nature 534, 378–382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.