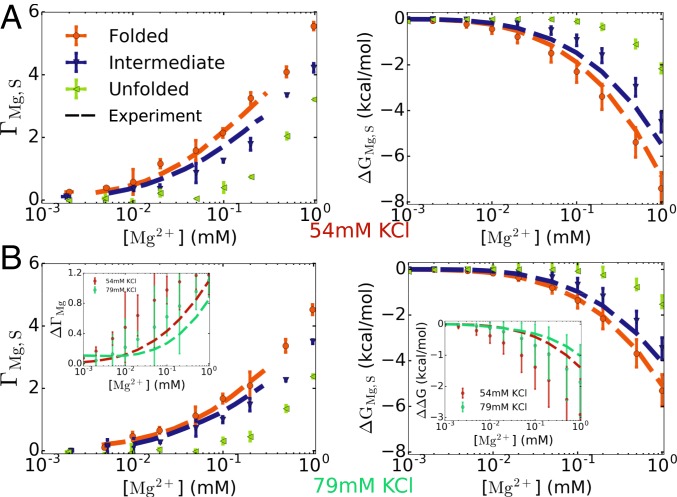
Fig. 3.
(A and B) Ion preferential interaction coefficient (Left) and free energy of Mg2+–pseudoknot interaction (Right) for BWYV in 54 mM KCl (A) and 79 mM KCl (B), at 25 °C. The calculations were performed for the folded, intermediate, and unfolded states (S = F, I, or U) (see main text and Fig. 4 for definition of the states). Experimental data for the F and I states are plotted as dashed lines (67). The results for the U state serve as a prediction of the simulations. The differences between the F and I states, and , are plotted in B, Insets. (Left Inset) represents the number of Mg2+ ions released when BWYV transitions from the F to the I state. (Right Inset) is the change in the free energy of the F state relative to the I state upon addition of Mg2+ ions. The error bars in Insets are relatively large. However, it is clear that and are not constant in the range of [Mg2+] here.