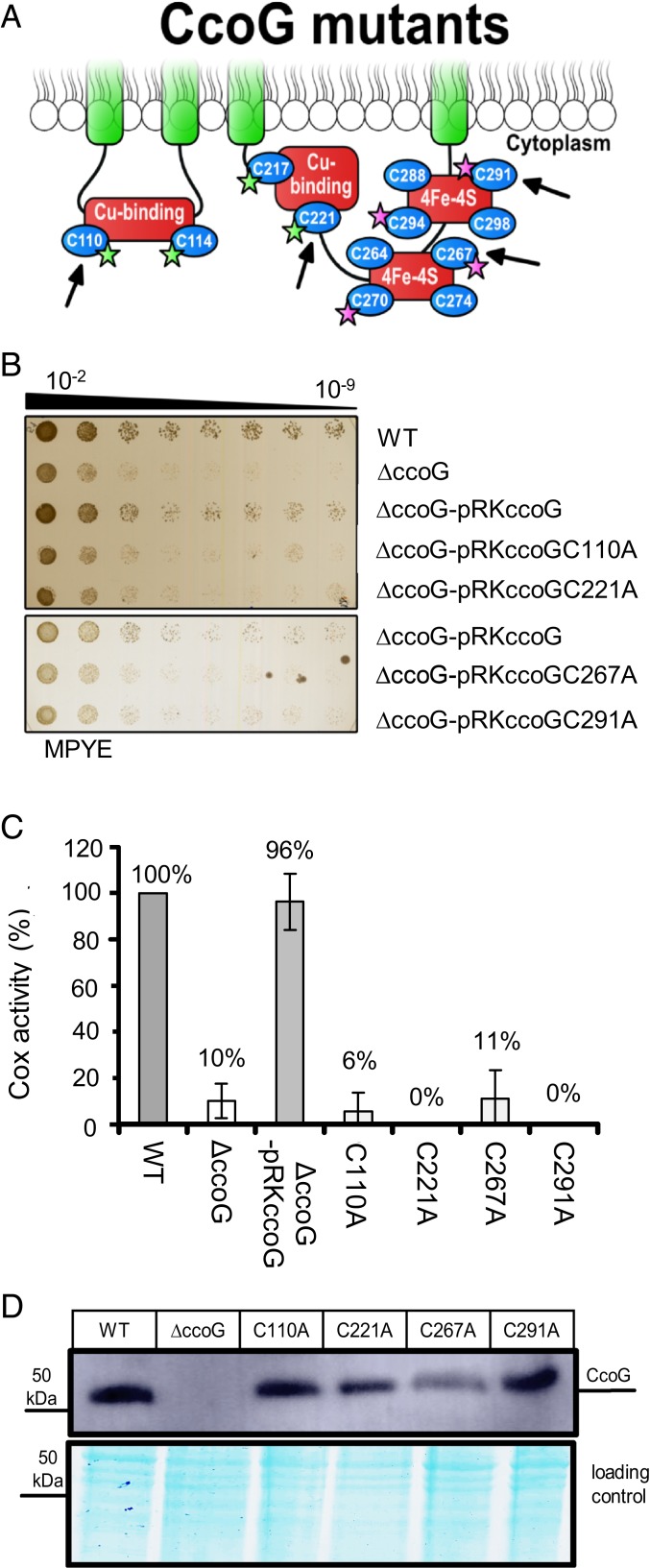
Fig. 4.
All 4 cysteine motifs are required for CcoG function. (A) A cartoon of CcoG showing its Cu and [4Fe-4S] cluster-binding motifs as red boxes. The single mutants were derivatives of pRKccoGFLAG in R. capsulatus (SI Appendix, Table S2) and the Cys-to-Ala mutations (C110A, C221A, C267A, and C291A) are indicated by arrows. The quadruple mutants were derivatives of pBADccoG in E. coli (SI Appendix, Table S2); the CcoG C110, C114, C217, and Cys221 residues replaced with Ser are indicated by green stars in the case of CcoG(ΔCu), and the C267, C270, C291, and C294 residues replaced with Ser indicated by purple stars in the case of CcoG(ΔFeS) are shown. (B) The strains described in A were tested for growth on enriched MPYE plates after incubation at 35 °C for about 20 h. A representative set is shown (n = 3). (C) The cbb3-Cox activity of the indicated strains was determined as in Fig. 3A. Each activity was measured at least twice in at least 3 independent experiments (n = 3). The cbb3-Cox activity exhibited by the wild-type strain was set to 100%. Statistical analysis was performed on the raw data using the Student’s t test with P < 0.0003 as the level of significance between the wild type and all strains, with the exception of the ΔccoG-pRKccoGC267A (P < 0.0007) and ΔccoG-pRKccoG (P > 0.05). Data are presented as means ± SD. (D) Immunoblot showing that the Cys-to-Ala substitution mutant derivatives of CcoG are produced in R. capsulatus (50 µg of membranes was loaded).