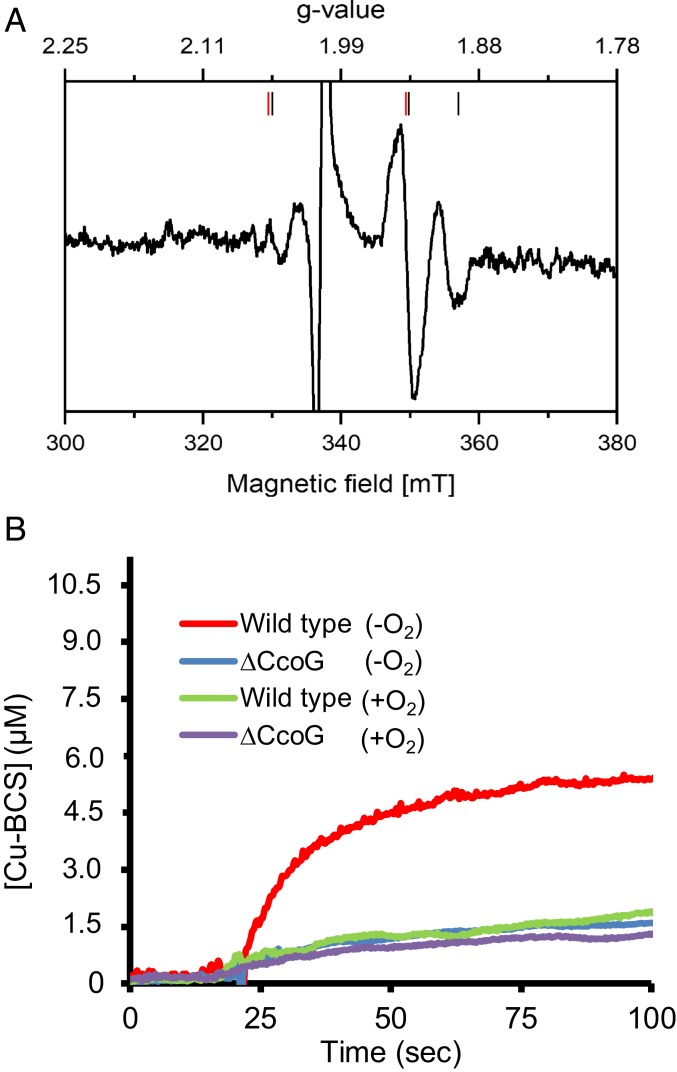
Fig. 5.
Membranes containing CcoG have Fe-S clusters and exhibit cupric reductase activity. (A) E. coli membranes isolated under anaerobic conditions from a strain overproducing CcoG and from one carrying the empty expression plasmid were reduced by addition of a few grains of dithionite, incubated for 30 s, and transferred into an EPR tube. EPR spectra were recorded at 13 K, 10-mW microwave power and with 10 scans. The spectrum obtained with membranes from the strain containing the empty expression plasmid was subtracted from that of the strain overproducing CcoG. The signals attributed to each cluster are indicated by red (axial) and black (rhombic) bars. The large radical signal at g = 2.0 due to dithionite reduction is omitted. Other EPR parameters used were: microwave frequency, 9.471 GHz; modulation amplitude, 0.6 mT; time constant, 0.164 s; scan rate, 17.9 mT/min. (B) Cupric reductase activity associated with CcoG was measured using anaerobically prepared R. capsulatus membranes from wild-type (MT1131) and ΔccoG (CW5) strains (SI Appendix, Table S2). In each case, 100 μg of membranes was mixed with 200 μM BCS anaerobically in a sealed cuvette, the assay was initiated by adding 20 μM Cu(II), and the production of the Cu(I)–BCS2 (Cu–BCS) complex was monitored at 482 nm. The kinetic difference seen between the wild-type and ΔccoG strains reflects the CcoG-dependent cupric reductase activity. As a control, the same assays were repeated after exposing the membranes to air (+O2) to inactivate the O2-sensitive CcoG-dependent cupric reductase activity. All experiments were repeated at least 3 times (n = 3) and a representative trace is shown.