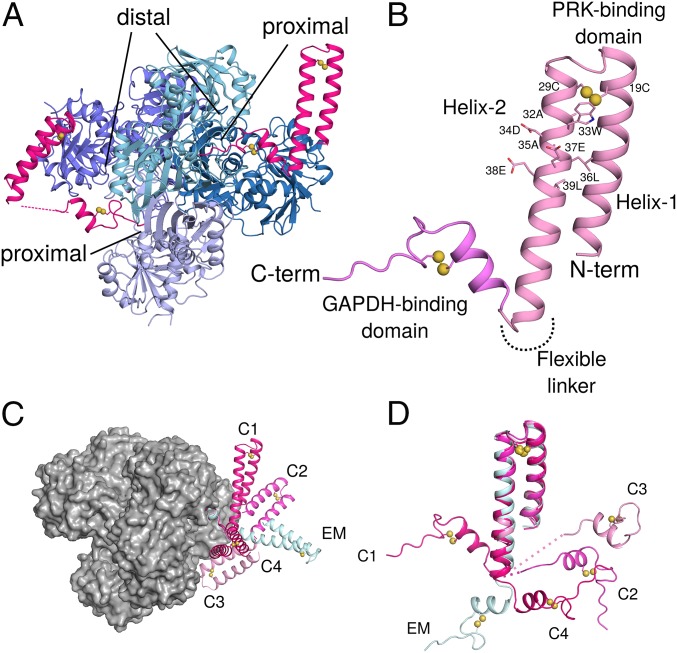
Fig. 2.
Crystal structures of GAPDH-CP12 complex with full-length CP12. (A) GAPDH tetramer (blue) with 2 active sites bound by CP12 (pink). Residues 55–75 of CP12 are inserted in the active site of GAPDH, while 1–55 form a 2-helix PRK binding domain. (B) CP12 is formed of 2 domains connected by a flexible linker. The N-terminal PRK binding domain is formed of 2 anti-parallel helices connected by a disulfide bridge at the apex of the helices. Residues of the conserved CP12 AWDA(V/L)EEL motif are shown as sticks; disulfide bonds are indicated by yellow spheres. The C-terminal GAPDH binding domain is formed of a 2-turn helical region followed by the remaining C-terminal residues that insert into the GAPDH active site. This fold is also stabilized by a disulfide bridge. (C) The different conformations of CP12 observed in crystals (C1–C4) and cryoEM (EM) superposed on the C-terminal region, bound to a GAPDH tetramer (gray surface). (D) CP12 conformations superposed on the N-terminal PRK binding domain.