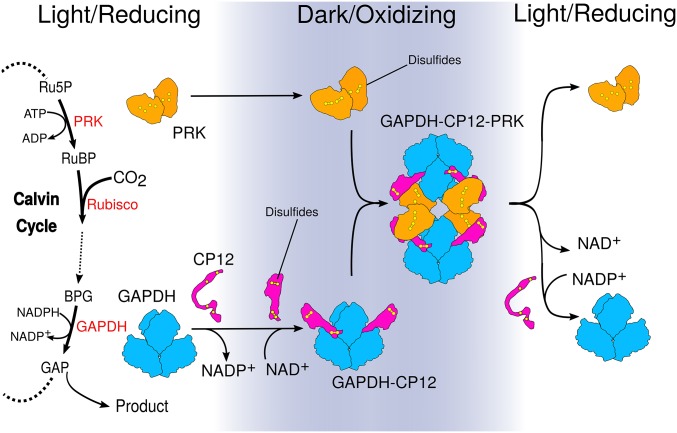
Fig. 4.
Model for how structural changes in redox-dependent complexes regulate carbon fixation. GAPDH and PRK are energy consuming enzymes of the Calvin–Benson cycle. The PRK catalyzed step directly precedes Rubisco carbon fixation. GAPDH (blue) activity is at the branch point between regeneration of RuBP or central metabolism. In the dark, the oxidizing environment causes intramolecular disulfide bridges to form within CP12 (pink) and PRK (orange). In parallel, NADP+ bound to GAPDH is exchanged for NAD+. CP12 binds to GAPDH, reducing its activity. Preordered CP12s subsequently recruit PRK, blocking PRK active sites to substrate and GAPDH active sites to NADP(H). When returning to the light, disulfide reduction dissociates the complex, releasing GAPDH and PRK.