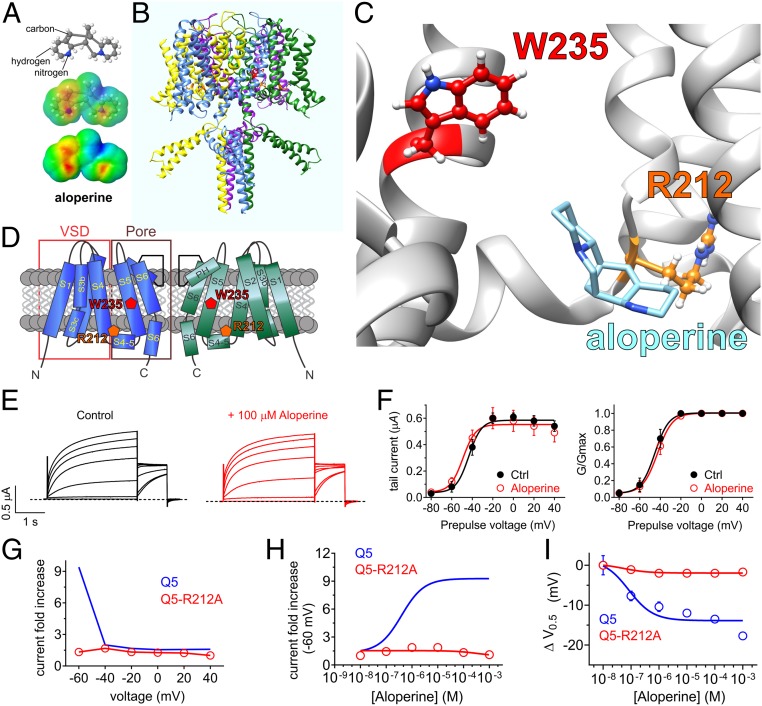
Fig. 6.
Aloperine activation of KCNQ5 requires KCNQ5-R212. All error bars indicate SEM. (A) Aloperine chemical structure (Upper and Center) and electrostatic surface potentials (red, electron-dense; blue, electron-poor; green, neutral; Lower and Center) calculated and plotted using Jmol. (B) Chimeric KCNQ1/KCNQ5 structural model (orange, KCNQ5-R212; red, KCNQ5-W235). (C) View of the aloperine binding site in KCNQ5 predicted by SwissDock (and see Fig. 7A). (D) Topological representation of KCNQ5 showing 2 of the 4 subunits, without domain swapping for clarity. Pentagons indicate approximate position of KCNQ5-R212 (orange) and KCNQ5-W235 (red). VSD, voltage sensing domain. (E) Mean TEVC current traces showing effects of aloperine (100 µM) on KCNQ5-R212 expressed in Xenopus oocytes (n = 5). (F) Mean tail current (Left) and mean normalized tail currents (G/Gmax; Right) versus prepulse voltage relationships for the traces as in E (n = 5). (G) Voltage dependence of effects of aloperine (100 µM) on channels indicated (from traces as in E and Fig. 2C; n = 5). (H) Aloperine dose response calculated from fold increase in current at −60 mV for channels as indicated (n = 5). (I) Aloperine dose response calculated from ΔV0.5activation for channels as indicated (n = 5).