Table 1.
Optimization of reaction conditionsa
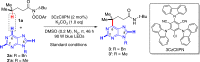
| ||
|---|---|---|
| Entry | Variation of reaction conditions | Yield(%)b |
| 1 | None | 89 |
| 2 | Without light | 0 |
| 3 | Air instead of N2 | 0 |
| 4 | Without K2CO3 | 16 |
| 5 | Without 3CzClIPN | 65 |
| 6 | Ir(ppy)3 instead of 3CzClIPN | 46 |
| 7 | Ru(bpy)3Cl2 instead of 3CzClIPN | 27 |
| 8 | Eosin Y instead of 3CzClIPN | 54 |
| 9 | 4CzIPN instead of 3CzClIPN | 67 |
| 10 | Na2HPO4 instead of K2CO3 | 78 |
| 11 | DIPEA instead of K2CO3 | 61 |
| 12 | CH3CN instead of DMSO | 27 |
| 13 | MeOH instead of DMSO | 0 |
| 14 | 45 W CFL instead of 90 W blue LEDs | 10 |
| 15c | 2′a instead of 2a | 76 (3′) |
aReaction conditions: 1a (0.2 mmol, 1.0 equiv), 2a (0.5 mmol), base (0.2 mmol), photocatalyst (2 mol%), solvent (1.0 mL), rt, 90 W blue LEDs
bIsolated yield
CTogether with 8% yield of C8-alkylated regioisomer. Ar = p-CF3C6H4
