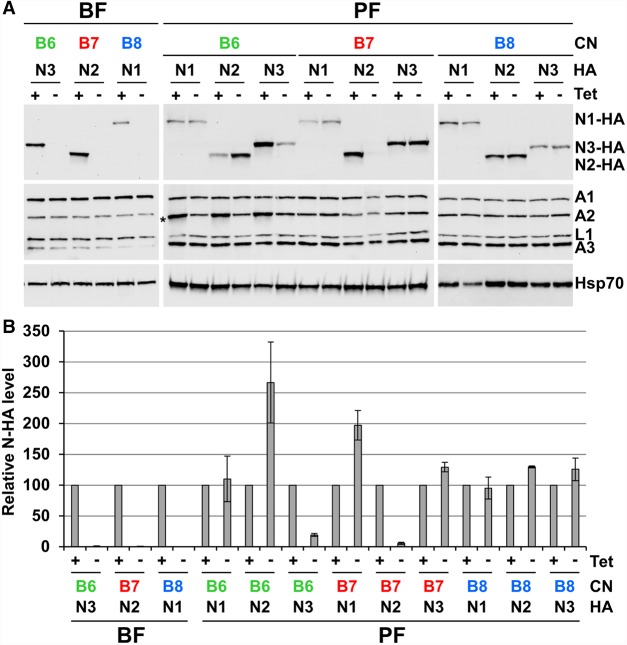
FIGURE 4.
Western blot analyses show KREPB6, KREPB7, and KREPB8 are critical for endonuclease abundance in BF and PF. The KREPB6, KREPB7, and KREPB8 CN cell lines were modified by introducing HA-epitope tags into the endogenous loci of each endonuclease. (A) Western analysis of total lysates (equivalent of 1 × 107 BF cells/lane, and 5 × 106 PF cells/lane) from BF and PF CN cells in which the tet-regulatable WT KREPB6, KREPB7, or KREPB8 alleles were expressed or repressed for 2 and 4 d, respectively. The western blots were probed with monoclonal antibodies against the HA-epitope tag on the cognate partner endonucleases, the editosome proteins KREPA1, KREPA2, KREL1, and KREPA3, and quantified by densitometry using mitochondrial Hsp70 for normalization. Asterisk denotes position of the TAP-tagged regulatable KREPB6 protein in PF, which is also detected by our editosome protein monoclonal antibodies. (B) Western quantification of the relative change in abundance of each endonuclease was determined in BF or PF CN cell lines that have either KREPB6, KREPB7, or KREPB8 repressed compared to the same cell line in which KREPB6, KREPB7, or KREPB8 was expressed. Data are shown as means ± SEM from two independent experiments (four experiments for PF KREPB6/KREN2-HA, KREPB7/KREN2-HA, and KREPB8/KREN1-HA).