Abstract
Background.
Obesity is associated with increased risk for various gastrointestinal and liver diseases. However, the relationship between obesity and abnormal bowel habits is poorly understood.
Aims.
To investigate the relationship between Body Mass Index (BMI) and bowel habit, controlling for clinical, demographic and dietary factors, in a representative sample of the United States adult population.
Methods.
Data were extracted from the 2009–2010 National Health and Nutrition Examination Survey (NHANES). Survey responses were included in this study if respondents completed the bowel health questionnaire (BHQ), were ≥20 years of age, and did not report history of inflammatory bowel disease, celiac disease, or colon cancer. BMI was divided into the following categories: underweight, normal weight, overweight, obese, and severely obese. Stepwise logistic regression provided risk ratios of constipation and diarrhoea controlling for confounding factors (dietary, life-style, psychological and medical).
Results.
A total of 5,126 respondents completed the BHQ, had BMI data available, and met eligibility criteria. Of these, 70 (1.40%) were underweight, 1,350 (26.34%) were normal weight, 1,731 (33.77%) were overweight, 1,097 (21.40%) were obese, and 878 (17.13%) were severely obese. Up to 8.5% of obese and 11.5% of severely obese individuals had chronic diarrhoea, compared to 4.5% of normal weight individuals. Stepwise regression revealed that severe obesity was independently associated with increased risk of diarrhoea.
Conclusions.
In conclusion, this study provides strong evidence that obesity is positively associated with chronic diarrhoea in a nationally representative US adult population after adjusting for several known confounding factors
Keywords: Obesity, diarrhoea, BMI
Introduction
The obesity epidemic continues to rise in the United States (US) and about 40% of the US adults were obese in 2015–20161. Obesity is associated with increased morbidity and mortality related to its cardiovascular complications2. However, it also increases the risk for various gastrointestinal and liver diseases. For example, obesity is a direct cause of non-alcoholic fatty liver disease and increases risk for gastro-esophageal reflux disease, Barrett’s esophagus and gall stones3. However, only a few studies have investigated the relationship between obesity and abnormal bowel habits.
In a population-based study from the US, the prevalence of diarrhoea in obese individuals was 30% compared to 17% in normal-weight controls4. Population based studies from other countries such as Australia, New Zealand and Sweden have also reported an association between obesity and chronic diarrhoea5–7. However, none of these studies were based on nationally representative samples and several had highly specific populations (e.g. the US study was based on a middle-aged Caucasian population4 and the study from New Zealand was based on a birth cohort aged 26 years5). Moreover, these studies did not report data on all BMI classes (underweight, normal BMI, overweight, obese, severe obese) and did not use the Bristol stool form scale (BSFS). The BSFS8, which combines picture with standardized descriptors of stool consistency, is now utilized by Rome criteria to define diarrhoea and is also one of the Federal Drug Administration recommended treatment outcome measures in diarrhoea predominant IBS (IBS-D)9. In addition, most of these studies lack data on confounding factors such as comorbid diabetes, psychological factors, and lifestyle factors, which are known to independently affect bowel habits5–7. Although these studies have postulated high carbohydrate and fat intake as the underlying etiology for chronic diarrhoea in obese individuals, these studies did not present data on dietary carbohydrate or fat intake5–7. Thus, it is unclear whether the association between obesity and diarrhoea is driven primarily by dietary factors.
In this study, we aim to investigate the relationship between BMI and bowel habit (diarrhoea, constipation and normal), controlling for clinical, demographic and dietary factors, in a representative sample of the United States adult population using the National Health and Nutrition Examination Survey (NHANES).
Materials and Methods
The National Health and Nutrition Examination Survey (NHANES) survey program is conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control (Atlanta, GA, USA). Participants are non-institutionalized individuals in the United States and are selected using stratified multistage probability design with oversampling of certain ethnic and age groups in order to allow for sample-weighted inference to the U.S. population. All participants provide written informed consent prior to completing the NHANES and there are no patient identifiers in the publicly available NHANES database.
Data were extracted from the 2009–2010 NHANES database. Participants in the NHANES 2009–2010 survey were included in this study if they completed the bowel health questionnaire, were at least 20 years of age, and did not report any history of having been diagnosed with inflammatory bowel disease, celiac disease, and/or colon cancer.
Bowel Health Questionnaire and Mood Questionnaires
Chronic diarrhoea and chronic constipation were identified based on responses to the bowel health questionnaire. Participants were shown a colored picture card with descriptions of the seven Bristol Stool Form Scale types (BSFS; Type 1-Type 7) and asked: ‘Please look at this card and tell me the number that corresponds with your usual or most common stool type’. Consistent with previous research10–12, chronic constipation was defined as a “usual or most common” stool type of BSFS Type 1 (separate hard lumps, like nuts) or Type 2 (sausage-like, but lumpy). Chronic diarrhoea was defined as a “usual or most common” stool type of BSFS Type 6 (fluffy pieces with ragged edges, a mushy stool) or Type 7 (watery, no solid pieces). Remaining subjects were classified as having normal bowel habits.
Obesity was defined based on standard Body Mass Index cutoffs. Body measurement data were collected for the NHANES in the Mobile Examination Center (MEC) by trained health technicians. Body Mass Index (kg/m2) was calculated based on the body measurement data. Obesity categories were defined as: underweight (BMI<18.5); normal weight (BMI between 18.5–24.9); overweight (BMI between 25.0–29.9); Obese (BMI between 30–34.9); Severely Obese (BMI >35).
Co-variables
A number of co-variables were evaluated as factors hypothesized or previously shown to associate with chronic diarrhoea and/or chronic constipation and obesity10,13. Calculations of adjusted risk ratios were performed following calculations of unadjusted risk ratios for all variables.
The co-variables included in this study comprised demographics/lifestyle, laxative use, and diet. Demographics/lifestyle factors included gender, age (divided into groups by decade: 20–29, 30–30, 40–49, 50–59, 60–69, and ≥70 years old), race (Non-Hispanic White, Non-Hispanic Black, Hispanic, and other race/ethnicity including multi-racial), education (“less than high school”, “high school or GED”, or “greater than high school”), poverty income ratio (grouped as less than two times the poverty income threshold or greater than or equal to two times the poverty income threshold), presence of depression (defined as a score of ≥10 on the PHQ-9), and physical activity in a typical week, divided into self-reported “vigorous” physical activity (defined as ‘vigorous-intensity activity that causes large increases in breathing or heart rate like carrying or lifting heavy loads, digging or construction work for at least 10 minutes continuously’) or “no vigorous activity”. Patients were considered laxative users if they reported having taken a laxative in the past 30 days. There were very low numbers of respondents taking other medications that may impact bowel function (e.g. anticholinergics) and, therefore, we did not control for other medications in our analyses.
Dietary factors included self-reported milk intake divided into four categories: never consume, rarely consume (less than once a week), sometimes consume (once a week or more, but less than once a day), and often consume (once a day or more) and self-reported alcohol intake: never drink, former drinker, rare drinker, light drinker, moderate drinker, and heavy drinker. Caffeine, fiber, liquid, carbohydrates, sugar, protein, and fat were all measured using gram values from dietary intake parameters obtained from the first day of a 24-hour recall period and divided into quartiles based on previous literature10.
To identify subjects with diabetes, the Diabetes Questionnaire from the 2009–2010 NHANES was used. Participants were asked to respond to the following: ‘Doctor told you have diabetes.’ Subjects who responded ‘Yes’ were classified as having diabetes. If subjects did not answer ‘Yes’ in response to “Doctor told you have diabetes,’ but responded ‘Yes’ to either ‘Taking insulin now’ or ‘Take diabetic pills to lower blood sugar,’ then they were also classified as having diabetes. Participants who answered ‘No’ in response to ‘Doctor told you have diabetes’ and did not answer ‘Yes’ in response to ‘Taking insulin now’ or ‘Take diabetic pills to lower blood sugar’ were considered non-diabetic subjects.
Statistics
All statistical analyses were calculated with sampling weights to account for the complex nature of the NHANES database’s survey design. Statistical analyses were performed using STATA statistical software version 14.2 (College Station, TX, U.S.A.).
Categorical variables were presented as frequencies and compared using chi-square tests. Stepwise logistic regression was used to determine the prevalence odds ratios of chronic constipation and chronic diarrhoea using the following models: Model 1 evaluated the unadjusted association between obesity categories (all compared to normal) and bowel habit; Model 2 evaluated the association between these obesity and bowel habit, controlling for demographic and lifestyle factors; Model 3 included obesity categories, demographic/lifestyle, and laxative use; Model 4, included obesity, demographic/lifestyle, laxative use, and dietary factors; and the final model, Model 5, controlled for a comorbid diagnosis of diabetes. Respondents with missing data in any of the variables included in each model were excluded from that model and subsequent models.
Results
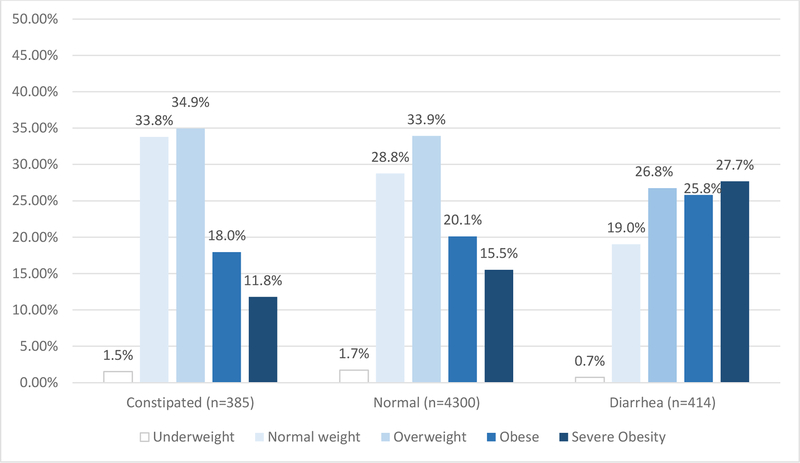
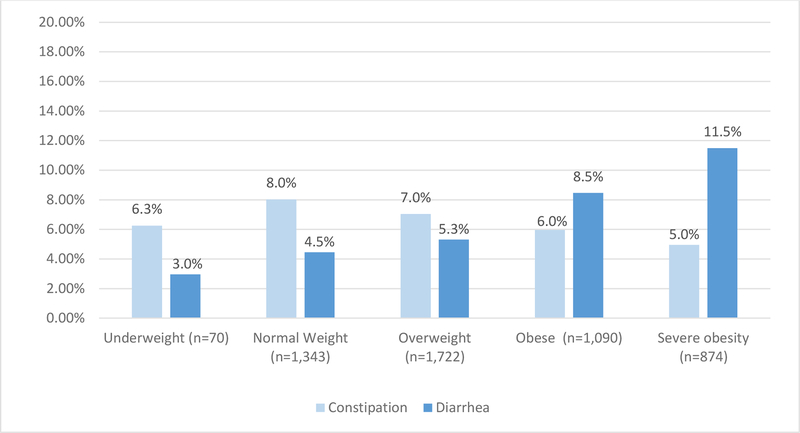
A total of 5,126 respondents met our eligibility criteria (see supplementary figure 1 for a flowchart of study participant eligibility/inclusion). Of these, 70 (1.40%) were underweight, 1,350 (26.34%) were normal weight, 1,731 (33.77%) were overweight, 1,097 (21.40%) were obese, and 878 (17.13%) were severely obese. In the weighted sample, a higher percentage of respondents with diarrhoea were obese or severely obese (25.82%, 27.68%, respectively) compared to normal bowel habits or constipation, while this was not the case for respondents in the overweight, normal, or underweight BMI categories. Similarly, up to 8.5% of obese and 11.5% of severely obese individuals had chronic diarrhoea, compared to 4.5% of normal weight individuals. (figure 1 and 2). Descriptive, univariate data for each co-variable is provided in supplementary table 1.
Figure 1.
BMI distribution within each bowel habit type (constipation, normal, diarrhoea)
Figure 2.
Percentage with diarrhoea or constipation within each BMI category.
Stepwise regression
Model 1 of the stepwise regression provides unadjusted risk ratios predicting constipation and diarrhoea for each BMI category compared to normal BMI (table 1). Obese and severe obesity were significantly associated with diarrhoea, with obese individuals nearly twice as likely and severe obese individuals nearly three times as likely to have diarrhoea compared to normal weight. No BMI categories were predictive of constipation in model 1.
Table 1.
Stepwise forward regression models predicting diarrhoea and constipation
| Diarrhoea | Constipation | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 (unadjusted)1 | ||||||||
| n=4,714 | n=4,685 | |||||||
| POR | 95% CI | p-value | POR | 95% CI | p-value | |||
| Underweight | 0.64 | 0.20 | 2.03 | 0.426 | 0.75 | 0.27 | 2.10 | 0.56 |
| Overweight | 1.19 | 0.91 | 1.57 | 0.190 | 0.88 | 0.63 | 1.22 | 0.41 |
| Obese | 1.94 | 1.29 | 2.93 | 0.003 | 0.76 | 0.49 | 1.18 | 0.21 |
| Severe obesity | 2.70 | 1.99 | 3.66 | <0.001 | 0.65 | 0.37 | 1.15 | 0.13 |
| Model 2 (adjusted for demographics/lifestyle)2 | ||||||||
| n=4,313 | n=4,288 | |||||||
| POR | 95% CI | p-value | POR | 95% CI | p-value | |||
| Underweight | 0.62 | 0.20 | 1.96 | 0.390 | 0.77 | 0.26 | 2.34 | 0.629 |
| Overweight | 1.03 | 0.77 | 1.37 | 0.845 | 1.06 | 0.77 | 1.46 | 0.690 |
| Obese | 1.73 | 1.06 | 2.83 | 0.030 | 0.84 | 0.50 | 1.42 | 0.494 |
| Severe obesity | 2.12 | 1.55 | 2.90 | <0.001 | 0.61 | 0.34 | 1.09 | 0.088 |
| Model 3 (adjusted for demographics/lifestyle and laxative use)3 | ||||||||
| n=4,310 | n= 4,286 | |||||||
| POR | 95% CI | p-value | POR | 95% CI | p-value | |||
| Underweight | 0.62 | 0.20 | 1.95 | 0.388 | 0.78 | 0.25 | 2.43 | 0.644 |
| Overweight | 1.02 | 0.76 | 1.37 | 0.880 | 1.02 | 0.74 | 1.40 | 0.918 |
| Obese | 1.73 | 1.06 | 2.83 | 0.031 | 0.81 | 0.48 | 1.36 | 0.393 |
| Severe obesity | 2.12 | 1.54 | 2.91 | <0.001 | 0.58 | 0.32 | 1.05 | 0.070 |
| Model 4 (adjusted for demographics/lifestyle, laxative use, and diet)4 | ||||||||
| n=4,260 | n= 4,235 | |||||||
| POR | 95% CI | p-value | POR | 95% CI | p-value | |||
| Underweight | 0.60 | 0.19 | 1.89 | 0.364 | 0.78 | 0.24 | 2.48 | 0.652 |
| Overweight | 0.98 | 0.71 | 1.35 | 0.884 | 1.00 | 0.74 | 1.35 | 0.997 |
| Obese | 1.70 | 1.05 | 2.74 | 0.033 | 0.75 | 0.47 | 1.19 | 0.209 |
| Severe obesity | 2.06 | 1.51 | 2.81 | <0.001 | 0.56 | 0.33 | 0.98 | 0.041 |
| Model 5 (adjusted for demographics/lifestyle, laxative use, diet, and comorbid diabetes)5 | ||||||||
| n=4,182 | n=4,160 | |||||||
| POR | 95% CI | p-value | POR | 95% CI | p-value | |||
| Underweight | 0.61 | 0.20 | 1.88 | 0.364 | 0.80 | 0.25 | 2.54 | 0.688 |
| Overweight | 0.99 | 0.72 | 1.37 | 0.976 | 1.00 | 0.74 | 1.34 | 0.970 |
| Obese | 1.61 | 1.00 | 2.61 | 0.050 | 0.75 | 0.50 | 1.13 | 0.158 |
| Severe obesity | 1.93 | 1.35 | 2.76 | 0.001 | 0.55 | 0.32 | 0.95 | 0.035 |
Model 1: unadjusted RRs for each BMI category compared to normal weight
Model 2: adjusting for demographics/lifestyle variables - Gender (female), age (decade), race (white), living above the poverty income threshold; higher education (at least some college); self-report of vigorous physical activity in a typical week; and presence of depression based on scores on the PHQ-9
Model 3: adjusting for demographics/lifestyle variables and laxative use (in the last 30 days).
Model 4: adjusting for demographics/lifestyle, laxative use, and the following diet variables - self-reported milk intake divided into four categories (high consumption, “once a day or more”), self reported alcohol intake (heavy), and highest quartile of caffeine, fiber, liquid, carbohydrates, sugar, protein, and fat.
Model 5: adjusting for adjusting for demographics/lifestyle, laxative use, dietary factors, and presence of comorbid diabetes
Risk Ratios for models 2 and 3, controlling for demographics/lifestyle and laxative use, are shown in Table 1. As in model 1, obesity and severe obesity were significant predictors of diarrhoea and no BMI categories were significantly associated with constipation.
Model 4 adjusts for demographics/lifestyle, laxatives, and dietary factors. In this model again, obesity and severe obesity were associated with significantly higher odds of having diarrhoea compared to those with normal weight. Additionally, severely obese individuals were approximately half as likely to have constipation compared to normal weight individuals.
Finally, Model 5 adjusted for the above co-factors as well as self-reported diagnosis of diabetes and/or self-reported use of medication to manage blood sugar. This model also revealed that severely obese individuals were nearly twice as likely to report diarrhoea (POR=1.93) and were half as likely to report constipation (POR=0.55) compared to individuals of normal weight.
Discussion
In this study, we have provided the most comprehensive evaluation of the relationship between BMI and bowel habits to date using a nationally representative sample in the United States. We found that over 80% of individuals with chronic diarrhoea in the general population were overweight, obese, or severely obese. The prevalence of diarrhoea increases gradually with BMI and up to 8.5% of obese and 11.5% of severely obese individuals had chronic diarrhoea, compared to 4.5% of normal weight individuals. This is lower than the prevalence reported in previous studies (18% to 33%)4–7 perhaps because previous studies included select groups of individuals.
After adjusting for demographic, psychological, lifestyle, laxative use, dietary factors and comorbid diabetes, obese individuals had 60% increased odds of having chronic diarrhoea compared to those with normal BMI. Similarly, after adjusting for above-mentioned variables, severely obese individuals had almost double the odds of having chronic diarrhoea compared to those with normal BMI. Thus, the relationship between obesity and chronic diarrhoea does not appear to be related to dietary factors and medical comorbidities alone. This is in agreement with our previous findings based on NHANES dataset which also showed an association between obesity and chronic diarrhea on multivariable analysis after adjusting for other variables of interest (such as diet, physical activity etc.). However, our previous study did not include various subgroups of obesity in the multivariable modeling. It also did not adjust for factors such as diabetes, and depression (based on PHQ-9 responses) both of which have been shown to be associated with chronic diarrhea.
Other studies investigating the relationship between diarrhoea and obesity have hypothesized that high carbohydrate and fat intake in obese individuals may be the underlying etiology for chronic diarrhoea4–7. However, none of those studies reported data on dietary intake. We found that the relationship between obesity and diarrhoea could not be explained by diet alone, as the significant association between the two persisted even after adjusting for intake of carbohydrates, sugars, and fat. Obesity is also associated with other medical comorbidities such as diabetes mellitus and depression, both of which are also associated with increased risk of chronic diarrhoea13,14. The previous studies on obesity and gastrointestinal symptoms did not take depression into account and only one accounted for diabetes5. We found that the association between obesity and diarrhoea in the general population is not entirely due to other comorbid conditions.
The exact etiology of chronic diarrhoea in obese individuals is not clear. However, a few studies have suggested that bile acid malabsorption is more common in obese individuals compared to those with normal BMI15,16. Obese individuals also have faster colonic transit compared to those with normal BMI17 and obesity is associated with increased intestinal permeability, microbial dysbiosis and endotoxemia (i.e. increased levels of lipopolysaccharide)18–21. Several studies have shown increased ratio of Firmicutes/Bacteroidetes in obesity, a finding also seen in diarrhoea predominant irritable bowel syndrome20,22. These physiological changes seen in obesity could be responsible for the relationship between obesity and diarrhoea.
This study has several strengths. Our study was based on a large, nationally representative sample of US adults with detailed data on demographic, lifestyle, dietary, psychological and medical comorbidities. Our assessment of diarrhoea was based on BSFS, which is a good correlate of colonic transit. However, our study does have a few weaknesses. Due to the study design, we were unable to evaluate the underlying etiology for the association between obesity and diarrhoea. We also did not have data on intake of fermentable carbohydrate intake such as high fructose corn syrup which has been linked with diarrhoea as well as obesity23. We defined constipation and diarrhoea based on responses to the Bowel Health Questionnaire, which includes the Bristol Stool Form Scale (a validated measure of stool consistency) but does not include any Rome diagnostic questions. As a result, we cannot be sure that the participants included here would meet full Rome criteria for a diagnosis of constipation or diarrhoea and we were unable to identify respondents with Irritable Bowel Syndrome. Finally, we acknowledge that obese individuals might take drugs and over the counter medications (such as stimulants, orlistat, green tea/coffee extracts) which could result in softer bowel movements. However, we did not have data on use of these drugs.
In conclusion, our study provides strong evidence that obesity is positively associated with chronic diarrhoea in a nationally representative US adult population after adjusting for several known confounding factors (dietary, life-style, psychological and medical comorbidities). The risk of diarrhoea increases with severity of obesity. Future studies should explore the underlying physiologic mechanisms of the association between obesity and chronic diarrhoea.
Supplementary Material
STROBE Statement—Checklist of items that should be included in reports of cross-sectional studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 2 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 3–4 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 4 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 4 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 4 |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants | 4 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 5–7 |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 5–7 |
| Bias | 9 | Describe any efforts to address potential sources of bias | NA |
| Study size | 10 | Explain how the study size was arrived at | 5,8 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 7 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 7 |
| (b) Describe any methods used to examine subgroups and interactions | 7 | ||
| (c) Explain how missing data were addressed | 7 | ||
| (d) If applicable, describe analytical methods taking account of sampling strategy | NA | ||
| (e) Describe any sensitivity analyses | NA | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | Supplementary material |
| (b) Give reasons for non-participation at each stage | NA | ||
| (c) Consider use of a flow diagram | |||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 8; supplementary table |
| (b) Indicate number of participants with missing data for each variable of interest | Supplementary table | ||
| Outcome data | 15* | Report numbers of outcome events or summary measures | 5–7 |
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 14 |
| (b) Report category boundaries when continuous variables were categorized | 5–6 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 8–9 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 9–10 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 11 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 11 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 9–11 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 1 |
Give information separately for exposed and unexposed groups.
Grant support:
This project was funded in part by NIH/NIDDK grant # T32DK007760 (SB, PS)
Footnotes
Disclosures: None
References
- 1.Center for Disease Control and Prevention. Adult Obesity Facts. https://www.cdc.gov/obesity/data/adult.html. Accessed July 1, 2019.
- 2.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 3.Camilleri M, Malhi H, Acosta A. Gastrointestinal Complications of Obesity . Gastroenterology. 2017;152(7):1656–1670. doi: 10.1053/j.gastro.2016.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgado-Aros S, Locke GR, Camilleri M, et al. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99(9):1801–1806. doi: 10.1111/j.1572-0241.2004.30887.x [DOI] [PubMed] [Google Scholar]
- 5.Talley NJ, Howell S, Poulton R. Obesity and chronic gastrointestinal tract symptoms in young adults: a birth cohort study. Am J Gastroenterol. 2004;99(9):1807–1814. doi: 10.1111/j.1572-0241.2004.30388.x [DOI] [PubMed] [Google Scholar]
- 6.Aro P, Ronkainen J, Talley NJ, Storskrubb T, Bolling-Sternevald E, Agréus L. Body mass index and chronic unexplained gastrointestinal symptoms: an adult endoscopic population based study. Gut. 2005;54(10):1377–1383. doi: 10.1136/gut.2004.057497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talley NJ, Quan C, Jones MP, Horowitz M. Association of upper and lower gastrointestinal tract symptoms with body mass index in an Australian cohort. Neurogastroenterol Motil. 2004;16(4):413–419. doi: 10.1111/j.1365-2982.2004.00530.x [DOI] [PubMed] [Google Scholar]
- 8.Lewis SJ, Heaton KW. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scandinavian Journal of Gastroenterology. 1997;32(9):920–924. doi: 10.3109/00365529709011203 [DOI] [PubMed] [Google Scholar]
- 9.Trentacosti AM, He R, Burke LB, Griebel D, Kennedy DL. Evolution of clinical trials for irritable bowel syndrome: issues in end points and study design. Am J Gastroenterol. 2010;105(4):731–735. doi: 10.1038/ajg.2010.12 [DOI] [PubMed] [Google Scholar]
- 10.Singh P, Mitsuhashi S, Ballou S, et al. Demographic and Dietary Associations of Chronic Diarrhoea in a Representative Sample of Adults in the United States. Am J Gastroenterol. 2018;113(4):593–600. doi: 10.1038/ajg.2018.24 [DOI] [PubMed] [Google Scholar]
- 11.Sommers T, Mitsuhashi S, Singh P, et al. Prevalence of Chronic Constipation and Chronic Diarrhoea in Diabetic Individuals in the United States. Am J Gastroenterol. November 2018. doi: 10.1038/s41395-018-0418-8 [DOI] [PubMed] [Google Scholar]
- 12.Markland AD, Palsson O, Goode PS, Burgio KL, Busby-Whitehead J, Whitehead WE. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am J Gastroenterol. 2013;108(5):796–803. doi: 10.1038/ajg.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballou S, Katon J, Singh P, et al. Chronic Diarrhoea and Constipation are More Common in Depressed Individuals. Clin Gastroenterol Hepatol. April 2019. doi: 10.1016/j.cgh.2019.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161(16):1989–1996. [DOI] [PubMed] [Google Scholar]
- 15.Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38(8):967–976. doi: 10.1111/apt.12466 [DOI] [PubMed] [Google Scholar]
- 16.Sadik R, Abrahamsson H, Ung K-A, Stotzer P-O. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol. 2004;99(4):711–718. doi: 10.1111/j.1572-0241.2004.04139.x [DOI] [PubMed] [Google Scholar]
- 17.Delgado-Aros S, Camilleri M, Garcia MA, Burton D, Busciglio I. High body mass alters colonic sensory-motor function and transit in humans. Am J Physiol Gastrointest Liver Physiol. 2008;295(2):G382–388. doi: 10.1152/ajpgi.90286.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491 [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernández-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE. 2012;7(5):e37160. doi: 10.1371/journal.pone.0037160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castaner O, Goday A, Park Y-M, et al. The Gut Microbiome Profile in Obesity: A Systematic Review. Int J Endocrinol. 2018;2018:4095789. doi: 10.1155/2018/4095789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie. 2016;124:11–20. doi: 10.1016/j.biochi.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajilić-Stojanović M, Biagi E, Heilig HGHJ, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1792–1801. doi: 10.1053/j.gastro.2011.07.043 [DOI] [PubMed] [Google Scholar]
- 23.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. doi: 10.1093/ajcn/79.4.537 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.