Abstract
Primary T cell immunodeficiency and HIV-infected patients are plagued by non-viral infections caused by bacteria, fungi and parasites, suggesting an important and underappreciated role for T lymphocytes in controlling microbes. Here we review recent studies showing that killer lymphocytes use the antimicrobial cytotoxic granule pore-forming peptide granulysin, induced by microbial exposure, to permeabilize cholesterol-poor microbial membranes and deliver deathinducing granzymes into these pathogens. Granulysin and granzymes cause microptosis, programmed cell death in microbes, by inducing reactive oxygen species and destroying microbial antioxidant defenses and disrupting biosynthetic and central metabolism pathways required for their survival, including protein synthesis, glycolysis and the Krebs cycle.
Graphical abstract
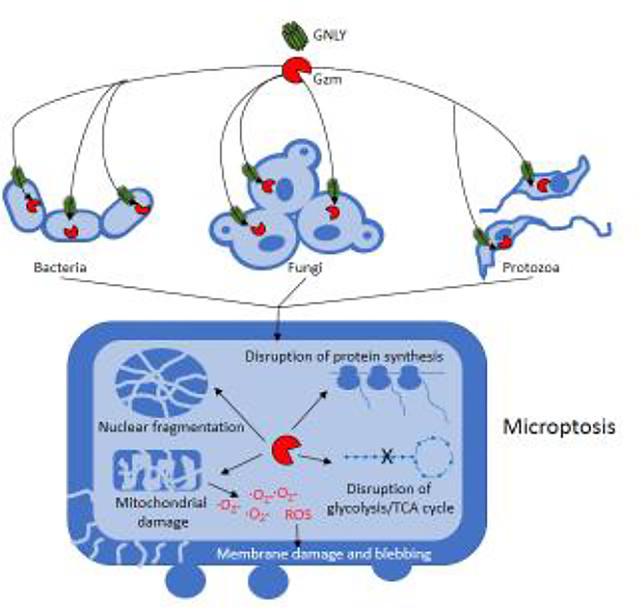
Introduction
When killer lymphocytes recognize infected or cancerous cells, they release cytotoxic granules that contain death-inducing proteins to cause programmed cell death that eliminates the infected or transformed cell without causing undue bystander death or inflammation [1]. Killer lymphocytes are well known to protect against virus infection and cancer by releasing cytotoxic granules and cytokines, including IFNγ and TNFα. However, patients with hereditary or acquired T cell immunodeficiency are highly susceptible to non-viral infections caused by bacteria (such as mycobacteria), fungi and parasites, suggesting that T lymphocytes might play an important role in microbial defense. NK cells respond immediately to infection by recognizing common features of infected target cells, such as perturbations of major histocompatibility protein expression or molecular signs of cellular stress, as part of innate immunity. Most killer T cells, as part of the adaptive immune response, only expand and become cytotoxic about a week after they first encounter target cells. However, innate-like killer T cells (γδ T cells, NK T cells, mucosal associated invariant T (MAIT) cells) that have restricted T cell receptors that recognize common features of infected cells, including pathogenic lipids and microbial metabolites, are often localized at barrier surfaces where infection enters the body. These killer cells may have been activated by other infections or commensal organisms in the past and many (like NK cells) are armed with cytotoxic proteins and stand ready for immediate defense against infection.
The cytotoxic granules of human killer cells contain two pore-forming proteins, perforin (PFN) and granulysin (GNLY), and 5 death-inducing serine proteases, called granzymes (granule enzyme (Gzm) A, B, H, K, M). PFN, a cholesterol-dependent cytolysin that is active in cholesterolcontaining host cell membranes, delivers the other granule proteins into cells, while GNLY is an antimicrobial peptide that is inhibited by cholesterol and much more efficiently permeabilizes microbial than mammalian membranes (Figure 1). Together these two pore-forming proteins can in principle disrupt any host cell or pathogen membrane. GNLY is expressed by most mammals, but not rodents, which may be why it has been largely ignored. Here we review recent studies that indicate an important role of GNLY and killer cells in protection from bacteria, fungi and parasites.
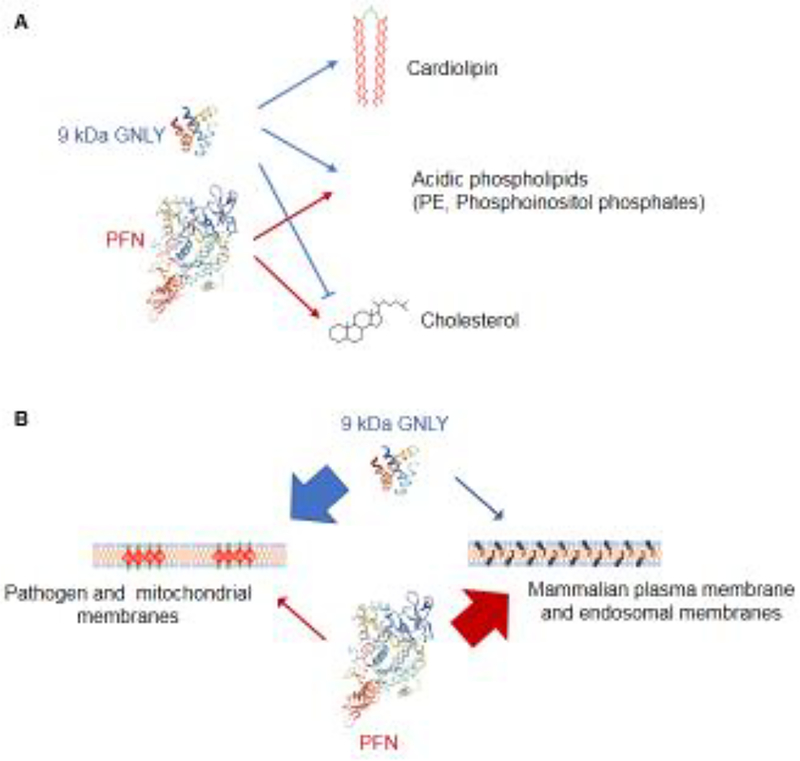
Fig. 1. GNLY and PFN permeabilize different membranes.
GNLY only works in cholesterol-poor membranes, while PFN requires cholesterol. Together they can permeabilize all cell membranes to deliver Gzms into both microbial and mammalian cells and potentially into multiple organelles. A Both GNLY and PFN bind to phosphoethanolamine (PE) and phosphoinositol phosphates, only GNLY binds to cardiolipin. B GNLY preferentially permeabilizes microbial membranes while PFN preferentially permeabilizes host cell membranes. However at high concentrations they have some activity on the other types of membrane.
Granulysin
GNLY was originally cloned as a late activation marker in T cells [2]. It is located on chromosome 2 of humans, has 5 exons and encodes for a 15 kDa precursor protein that is expressed only in killer cells (Figure 2). There is no evidence that the few common single nucleotide polymorphisms in the promoter and coding regions of human GNLY affect GNLY expression or function. No GNLY genetic deficiencies have been described. GNLY belongs to the saposin-like protein (SAPLIP) [3] family that bind to lipid membranes, have a common 4 α-helical structure with little amino acid homology, suggesting independent evolution towards a common function. They include poreforming proteins in amoebae (that kill phagocytosed bacteria) and vertebrate GNLY orthologs. Proteins with significant homology to GNLY have been described throughout vertebrate evolution, but notably not in rodents. Pore formation by these GNLY homologs, although likely, has only been experimentally demonstrated for human, pig and cow GNLY.
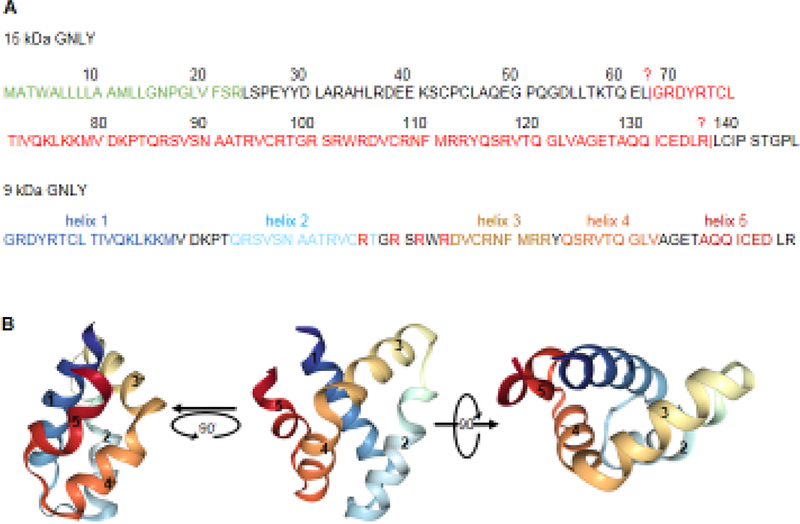
Fig. 2. GNLY sequence and structure.
A Full length 15 kDa GNLY pro-peptide has an N-terminal 23 aa signal peptide (green). An unknown enzyme cleaves the N and C terminal peptides after L62 and R136 to produce the 9 kDa active GNLY (red). B 9 kDa GNLY structure (1L9L) from the Protein Data Bank. 9 kDa active GNLY has 5 alpha helices of which helices 2 and 3 and the arginines (R) in the loop in between are most important for activity.
GNLY expression is induced by T cell activation, common γ-chain binding cytokines (especially IL-2, IL-15 and IL-21) and exposure to bacteria and other microbes, consistent with its postulated role in antimicrobial immunity [4]. GNLY enhancer and promoter regions and transcription factors that regulate its expression have not been mapped (except for a Stat5-binding site in a distal element presumed to be an enhancer [5]). The microbial molecular components (PAMPs?) or their receptors (TLRs?) responsible for microbial induction of GNLY are also unknown. In one paper a small N-terminal lipopeptide from a M. leprae lipoprotein stimulated dendritic cells to secrete IL-12 and induce GNLY expression in cocultured T cells [6], suggesting that recognition of microbial PAMPs may trigger GNLY expression. GNLY is highly expressed in killer cells in the skin and in decidual NK cells (dNK) in the placenta during the first trimester of pregnancy, again suggesting a role in protecting the barrier epithelia from infection [7–11].
GNLY is processed in killer cells by proteolytic removal of peptides from both the N- and C-termini to generate a 9 kDa active pore-forming protein that retains helices 2 and 3. The 15 kDa inactive full length pro-peptide is 145 aa long with a 23 amino acid signal sequence, while the active 9 kDa peptide is 74 aa long (from G63 to R136) (Figure 2). The 9 kDa enzyme is only active at neutral pH. The processing enzymes or where processing occurs are not known. Other cytolytic proteins are processed by cathepsins in cytotoxic granules and an inhibitor of cytotoxic granule acidification blocks GNLY processing, suggesting that GNLY processing to its active form likely occurs in cytotoxic granules. Proteolytic activation and storage of GNLY within acidic cytotoxic granules (which are maintained at a pH in which GNLY is inactive) would protect the killer cell from GNLY’s toxic effects on membranes. GNLY is largely confined to cytotoxic granules, although it can also be found in the cytosol in very highly expressing cells, such as activated peripheral blood NK cells and decidual NK cells in the placenta (A. Crespo, S. Mulik and J. Lieberman, unpublished data).
The molecular basis of GNLY pore formation is unclear. Like other cytotoxic granule proteins, GNLY is cationic (predicted pI of 9 kDa active protein is 10.3). GNLY permeabilizes liposomes that contain acidic phospholipids [12]. A patch of 4 basic Arg residues between the two critical amphipathic alpha helices (Figure 2) is postulated to mediate initial membrane binding and GNLY dimerization is also thought to be important in pore formation. However, GNLY pores have not been visualized and the size of the membrane channel and number of monomers that form the pore are not known. GNLY binds to immobilized phosphatidylinositol phosphates, phosphatidylethanolamine and cardiolipin [13] and is inhibited by cholesterol, which is abundant in eukaryotic plasma membranes. It is not known how cholesterol inhibits GNLY membrane damage or facilitates pore formation by cholesterol-dependent cytolysins (including bacterial toxins like perfringolysin or the mammalian complement membrane attack complex or the cytotoxic granule pore-forming protein perforin), but cholesterol decreases membrane fluidity. PFN binds to the same membrane lipids as GNLY, but doesn’t bind to cardiolipin. Cardiolipin is present only on mitochondrial and microbial (bacteria, fungi, parasites) membranes and could be especially important for GNLY pore formation in microbial membranes (Figure 1). Since GNLY binds to cardiolipin, if active GNLY leaks into the cytoplasm of killer cells or once in the cytosol of target cells under killer cell attack, it might also bind to and damage host cell mitochondrial membranes since mitochondrial membranes resemble bacterial membranes and are cholesterolpoor and rich in cardiolipin.
Granulysin in human health and disease
Killer cells that express high levels of GNLY, such as decidual NK cells, constitutively secrete the active protein into culture medium (to levels of ~1 nM = 9 ng/ml). However, in healthy donors GNLY in plasma is <10 ng/ml (2.5±0.2 ng/ml in one study [14]). The mean level of serum GNLY in patients with acute, relapsed or chronic tuberculosis, an infection in which GNLY is likely to have a role, was significantly lower than that in healthy donors, but in some individuals was slightly elevated, but in all cases <10 ng/ml. In healthy donors, very few circulating T cells stain above background for GNLY, even though a substantial minority (~25%) are memory cells that stain for other granule proteins including PFN and GzmB. However, small populations of circulating γδ T cells and NK (usually <20%) stain for GNLY, while virtually all NK cells and close to half of γδ T cells in the blood stain for PFN. Thus, GNLY expression is more closely regulated than expression of other cytotoxic proteins. In leprosy the expansion of CD8 T cells co-expressing GNLY, PFN and granzyme B is linked to disease control; these antimicrobial CD8 T cells also express many NK cell surface receptors and can be identified by staining for the activating receptor NKG2C [*15]. Similarly the subset of decidual NK cells that highly express GNLY, also express high levels of GzmA, GzmB and PFN and NK receptors, including NKG2C. The inhibitory NK receptors on decidual NK cells recognize HLA-C, E and G expressed by fetal trophoblasts, which help maintain tolerance of fetal cells in the placenta [16]. In the skin in some autoimmune and inflammatory conditions (alopecia areata, psoriasis), GNLY can be so strongly induced that it can cause keratinocyte death, skin blistering and sloughing, serious toxicity and even death (Figure 3)[9,10,17–20]. In patients who have severe drug reactions (Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN)) GNLY can reach concentrations of 50 μg/ml (~5 μM) in serum and blister fluids (in the same samples GzmB levels are 1000-fold less). These are the highest levels of GNLY recorded in humans.
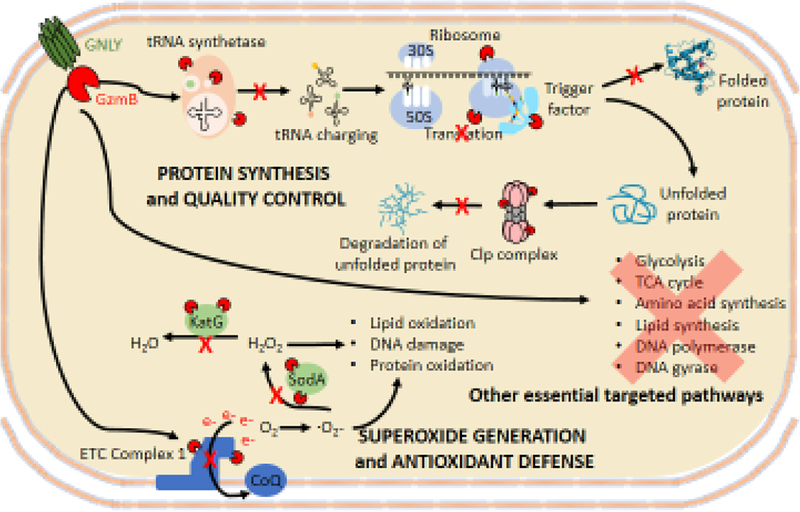
Fig. 3. Microptosis in bacteria.
GzmB, delivered into bacteria by GNLY, cleaves multiple substrates in essential metabolic and synthetic pathways to kill diverse bacterial strains and prevent bacterial resistance. Red Pacmen indicate GzmB cleaving substrates and red Xes indicate disrupted pathways. Generation of superoxide radicals and disruption of antioxidant defenses and disruption of protein synthesis and quality control are microptosis pathways that have been experimentally verified
Why don’t rodents have GNLY?
It is unclear why rodents, but not other mammals, have deleted GNLY. We can only speculate. A plausible explanation for why rodents deleted the GNLY gene could be that GNLY skin toxicity was too great a price to pay in exchange for better microbial protection. Another possibility is that the exceedingly high levels of GNLY in decidual NK cells, which might be increased even more during inflammation or infection, could lead to placental damage and pregnancy loss under some conditions. Another possibility is that other antimicrobial peptides, possibly produced by other types of cells, such as myeloid cells or epithelial cells, might deliver Gzms or other proteases into microbes to kill intracellular or cell-free microbes, providing a parallel pathway for anti-microbial defense.
Granulysin is an antimicrobial peptide that disrupts microbial membranes
Twenty years ago a pioneering paper showed that GNLY on its own kills a wide spectrum of cell free bacteria, including Mycobacteria tuberculosis (MTB), 2 fungi (C. albicans and C. neoformans) and a parasite (L. major) [21]. GNLY was found to cause blebbing of MTB membranes and swelling of the periplasm by electron microscopy and to permeabilize bacterial membranes [21–23]. Microbial killing required very high GNLY concentrations (>1 μM) - a concentration that is rarely reached in extracellular fluids in vivo except in severe, life-threatening skin reactions. It is currently unclear whether secreted GNLY, in contrast to GNLY released from cytotoxic granules by killer cells directly into immune synapses, has any physiological antimicrobial role. Negligible numbers of macrophages were killed even when incubated with 25 μM GNLY, suggesting that GNLY is selective for microbial membranes (Figure 2C). However, GNLY can bind to cholesterolpoor lipid rafts in mammalian cell membranes and is actively endocytosed into endosomes and can lyse intracellular Listeria in phagosomes [24]. However, GNLY endocytosis does not kill the mammalian cell [23]. Unlike perforin [25], endocytosed GNLY is unlikely to form pores efficiently in endosomal membranes (which resemble the plasma membrane and contain cholesterol) to get released into the target cell cytoplasm. In fact, killing of intracellular Listeria is greatly enhanced in the presence of a sublytic concentration of PFN (not enough to kill host cells on its own, but enough to deliver other cytotoxic granule proteins into cells) [26]. Moreover, intracellular mycobacteria within macrophages are killed only if infected cells are incubated with both PFN and GNLY [21].
Killer cells not only kill infected cells, but also reduce intracellular microbe counts
The role of killer cells in defense against microbial pathogens was first studied for mycobacteria. CD4 and CD8 killer T lymphocyte lines from healthy donors that are PPD immunoreactive recognize and kill intracellular MTB via cytotoxic granule exocytosis [27,28]. Infiltration of GNLY- expressing CD4 T cells that lyse M. leprae-pulsed macrophages and reduce mycobacterial colony formation into tuberculoid leprosy lesions is strongly correlated with localized disease [20]. However, the most abundant MTB-specific killer cells in MTB-infected patient blood are γ9δ2 T cells that recognize phosphoantigen intermediates of isoprenoid biosynthesis produced by bacteria and parasites, which are presented by the immunoglobulin-like B7 family molecule, butyrophilin 3A1 [29–31]. Vγ9Vδ2 T cells are only found in humans and primates (again not in mice!) and represent a very small proportion of peripheral blood lymphocytes but can expand dramatically during acute bacterial and parasite infections. NKT cells that express an invariant Vα24 T cell receptor that recognizes lipid antigens presented by a CD1 molecule are another innate-like killer cell type that recognize MTB-infected macrophages, degranulate and have antimycobacterial activity. These innate-like T cells reduce bacterial growth in a GNLY-dependent mechanism using the cytotoxic granule pathway. γ9δ2 T cells are also expanded in blood stageP. falciparum patients and are able to reduce parasite reinvasion in a GNLY-dependent manner [32,33]. Of note, the only correlate of protection in humans from the partially effective malaria circumsporozoite vaccine is γ9δ2 T expansion in the blood, suggesting that these innate-like T cells are important in malaria defense [34].
Cytotoxic lymphocytes have also been shown to kill fungi that have not invaded mammalian cells. NK cells, some of which express GNLY, and IL-15 activated CD8 T cells that up-regulate GNLY acquire cytotoxic granule-dependent anti-cryptococcal activity [35–37]. Surprisingly some killer lymphocytes, including CD4+ T cells and NK cells, have been shown to directly bind to cell-free fungi and kill them in a GNLY-dependent manner [38,39]. The NK activating receptors, NKp30 and NKp46, recognize fungal surface molecules and may act as fungal PAMP receptors that mediate binding and degranulation [40–43].
Thus, a variety of killer cells (CD4 and CD8 T cells, NK cells, NKT and γδ T cells) can inhibit intracellular infection in a GNLY-dependent manner by recognizing infected cells and releasing cytotoxic granules. None of these studies examined what happens to microbes within target cells undergoing killer cell attack. The reduction in infectious microbial colonies in these studies could have been the result of direct microbial killing and/or the destruction of the favorable cellular niche in which these microbes replicate.
Granulysin lysis of mammalian cells
GNLY has about a thousand-fold less activity permeabilizing cholesterol-rich mammalian cell membranes than microbial membranes. However, injection of the high concentrations of (even inactive15 kDa) GNLY released by skin CTL and NK cells into the blister fluids of patients suffering from severe skin drug reactions (SJS and TEN) on its own causes keratinocyte death and disease pathology [10]. Moreover, clinical severity, which can be fatal, correlates with GNLY levels in blister fluids. The in vivo importance of GNLY can be evaluated in GNLY transgenic (GNLY-Tg) mice that were engineered using a large bacterial artificial chromosome with large regions of 5’ and 3’ GNLY regulatory sequences [44]. As a consequence, GNLY in these mice is expressed only in killer lymphocytes at levels comparable to those in humans [44,45]. T cell lines from GNLY- Tg mice kill allogeneic mouse tumor cells significantly faster than their littermate control WT cells, but the overall amount of killing is not different. GNLY expression also hads no effect on NK killing. When these mice were injected with C6VL lymphoma cells, 20% of GNLY-Tg mice survived a lethal challenge that killed all WT mice, but the GNLY-Tg did not improve the survival of mice challenged with another lymphoma (RMA-S). Thus, the effect of GNLY on tumor cells is probably modest.
In one study [12] incubation of Jurkat cells with 50 μM GNLY (a higher concentration than has ever been measured in humans) killed 50% of Jurkat cells by disrupting membrane integrity. GNLY at these high concentrations also appeared to cause caspase-independent mitochondrial damage in cells (cytochrome c release, loss of transmembrane potential), which may not be surprising since mitochondrial membranes resemble bacterial membranes. It is also possible that other organelle membranes that have low cholesterol content compared to the plasma membrane, such as the Golgi, ER or lysosomes, might be damaged by GNLY in target cells [46,47]. Lysosomal membranes are also damaged to release cathepsins and cause proteolytic necrotic death when cells are incubated with 50 μM GNLY [48].
These results suggest that under most circumstances GNLY contributes weakly to killer cell lysis of mammalian cells. GNLY might work at physiological concentrations by potentiating the cytolytic activity of perforin and the granzymes. However, in conditions in which GNLY is superinduced it may become cytotoxic for mammalian cells. It will be worthwhile in the future to investigate more fully whether and how GNLY affects organelle membrane integrity and function in targets undergoing killer cell attack.
Granulysin delivers granzymes to cause microbial programmed cell death
During killer cell-mediated death PFN membrane damage of mammalian cells on its own does not kill cells since the PFN damage is transient and repaired by a ubiquitous plasma membrane repair pathway. Instead PFN facilitates cell death by delivering the death-inducing Gzm proteases into the target cell [25,49]. In target cells the granzymes cleave a number of cytosolic proteins, but also concentrate in and cleave critical substrates in nuclei and mitochondria. The similarity of microbial membranes to mitochondrial membranes led us to hypothesize that GNLY might introduce Gzms into intracellular microbes to directly kill them, much like PFN introduces Gzms into host cells. In fact sublytic nanomolar concentrations of GNLY, much lower than the concentrations used in the previously discussed studies, deliver Gzms into the cytosol of a variety of gram positive and gram negative cell-free bacteria to kill them [50,**51]. Killing of intracellular bacteria requires not only GNLY and a Gzm (both GzmA and GzmB are equally active but other Gzms have not been studied), but PFN to deliver the other molecules into the host cell. Killing of bacteria in cells occurs rapidly before the host cell, requires all 3 types of cytotoxic granule proteins (GNLY, PFN and a Gzm) and is independent of host cell killing, which is not enhanced by GNLY. Mouse CTL control of intracellular bacteria is abrogated in Prf1−/− cells and potentiated by GNLY-Tg, suggesting that killing the host cell reduces bacterial replication, but direct killing of bacteria potentiates antimicrobial activity.
When the Gzms enter bacteria, they cleave proteins in Electron Transport Chain complex I (ETC I) to disrupt oxidative phosphorylation and generate superoxide anion, as previously described in host cell mitochondria when Gzms enter the mitochondrial matrix [50,52–54] (Figure 3). In fact, the core proteins in electron transport that are targeted by the Gzms in mitochondria evolved from bacteria. Because microbes need to survive in many diverse and stressful environments, they have highly elaborated antioxidant defenses. Very strong oxidants, such as rotenone or paraquat, are bacteriostatic but do not kill bacteria. While generating reactive oxygen species, GzmB also cleaves and inactivates the key bacterial antioxidant enzymes, the superoxide dismutases and catalases, to cause bacterial death [50]. Rapid microbial death is inhibited by ROS scavengers and overexpressing antioxidant enzymes, demonstrating that killer cells orchestrate an oxidative death of bacteria.
Intracellular protozoan parasites (Trypanosoma cruzi, Toxoplasma gondii, Leishmania major) are also killed by killer cells [*45]. These parasites also undergo an oxidative death that is inhibited by ROS scavengers and overexpressing antioxidant enzymes. Like bacterial death, parasite death requires PFN to deliver GNLY and the Gzms and is faster than host cell death (limiting parasite spread when the host cell is killed). GzmB also inactivates antioxidant enzymes in T. cruzi. Within targeted cells parasites undergo cell death that morphologically resembles mammalian cell apoptosis in that mitochondria swell and lose their characteristic morphology, chromatin condenses and nuclei fragment and the parasite membrane blebs. However, parasite death is independent of either caspases or paracaspases. A recent study also shows that CD8 T cells, which are expanded and activated and express GNLY in patients with blood stage Plasmodium vivax malaria, also kill intracellular P. vivax parasites in red blood cells (RBC) to prevent invasion of fresh blood cells [*55]. CD8 T cells were previously not thought to recognize RBC, but this strain of malaria infects immature RBC reticulocytes. HLA class I is induced by malaria infection of reticulocytes, which CD8 T cells recognize. Killing of malaria parasites inside reticulocytes and lysis of infected reticulocytes does not require PFN. Malaria parasites harvest cholesterol from the RBC membrane, rendering it susceptible to GNLY. Nonetheless parasite killing is enhanced by PFN. Falciparum malaria infects mature RBC, which do not express HLA. Hence CD8 T cells do not recognize P. falciparum-infected RBC. However, they have previously been reported to be targeted by γδ T cells [32]. Our preliminary work confirms this finding and shows that GNLY and GzmB and γδ T cells also lyse P. falciparum-infected RBC and directly kill this fulminant strain.
Microptosis, transkingdom microbial programmed cell death
Treatment of bacteria with ROS scavengers or overexpressing pathogen oxidative defense enzymes slows down GNLY and Gzm mediated death but doesn’t prevent it, suggesting that other microbial pathways are also targeted [*45,50,**51]. GzmB substrates in 3 unrelated bacterial pathogens (L. monocytogenes, E. coli and MTB) were identified using differential proteomics [**51]. A few hundred candidate substrates were identified in each species (about 5–10% of resolved proteins). A subset of these candidate substrates were experimentally tested and ~90% were experimentally validated, suggesting that the proteomics used accurately predict substrates. Many of the substrates were common orthologues in all 3 bacteria that act in essential protein synthesis and central metabolism pathways, including glycolysis, the TCA cycle, oxidative phosphorylation, nucleotide, amino acid, and fatty acid biosynthesis, the oxidative stress response, DNA polymerase, gyrase and protein synthesis, folding and quality control (Figure 3). How the Gzms “know” to selectively cleave critical enzymes essential for microbial survival is unclear.
Protein synthesis and processing is a dominant shared pathway targeted in bacteria. Most antibiotics target protein synthesis at different steps including translation initiation (linezolid), aminoacyl tRNA binding (tetracyclines), peptidyl transfer (chloramphenicol, macrolides, quinupristin), ribosomal translocation (macrolides, aminoglycosides, fusidic acid) and termination (macrolides, puromycin, streptogramins), aminoacyl tRNA synthesis (mupirocin) and ClpP activation (acyldepsipeptide). GzmB cleaves multiple tRNA synthetases, ribosomal proteins, chaperones of newly synthesized peptides and the Clp system that degrades misfolded proteins. It simultaneously disrupts all these stages of protein synthesis and therefore acts like an antibiotic cocktail.
This concerted onslaught by cytotoxic granule effector proteins on essential microbial pathways triggers microbial programmed cell death, which is termed “microptosis” to distinguish it from death due to membrane damage caused by GNLY on its own (“microbe necrosis”).
Can microbes become resistant to microptosis?
Because Gzms disrupt so many critical pathways at the same time during microptosis, it may be nearly impossible for bacteria to develop resistance by mutating small numbers of genes. In fact, resistance did not develop when E. coli were serially passaged for 14 cycles in the presence of GNLY and GzmB [**51]. However, microorganisms could become resistant to GNLY, for example by reducing GNLY binding to microbial membranes by interfering with cardiolipin biosynthesis by mutating the genes encoding for cardiolipin synthase or by developing an impermeant capsule or cell wall in spores or growing cells to block GNLY and Gzm access to the cell membranes. In fact, increasing capsule thickness or melanization of cryptococcal capsules reduced, but did not eliminate, GNLY and GzmB killing of cryptococcus (F. Dotiwala and J. Lieberman, unpublished). Although bacteria are unlikely to become completely resistant to microptosis if Gzms get through the cell membrane, they could become less sensitive to Gzms and GNLY or take longer to kill. Mechanisms that could lead to relative insensitivity to GNLY and Gzms include overexpressing antioxidant enzymes, turning off aerobic metabolism or adapting a dormant state. In addition, certain types of growth conditions or certain types of bacteria (i.e. anaerobes) may be less sensitive or more likely to develop relative resistance. Although we have yet to identify microbes that completely escape Gzms and GNLY, additional work is needed to determine whether some microbes are intrinsically resistant to microptosis or can develop resistance and to identify resistance mechanisms. Of course, intracellular pathogens could also manipulate the host cell to evade killer cell recognition or activation by, for example, down-regulating cell surface expression of TcR or NK activating receptor ligands.
GNLY protection from in vivo infection
Clinical studies of humans infected with mycobacteria and malaria described above hint that GNLY expression in certain types of killer lymphocytes correlates with better outcome [14,56,57]. Moreover, some recent studies suggest that in the setting of chronic or relapsing infections, including HIV, MTB and malaria, GNLY expression is reduced [58,59], coinciding with other signs of killer cell dysfunction or exhaustion. Higher GNLY mRNA in cancer patient CD8 T cells is also associated with better survival [60,61]. This association does not necessarily mean that GNLY contributes to antitumor immunity but could just be an indicator of less T cell exhaustion. Mice expressing transgenic human GNLY are less susceptible to bacterial and parasite infections than WT mice [*45,50]. Because mice lack GNLY and a number of other human immune genes that mediate killer cell recognition or function, caution should be used when applying information about pathways required for immune protection in mouse models to human infection.
Because GNLY is not expressed in rodents, GNLY-Tg mice, crossed or not with mice deficient in individual cytotoxic enzyme genes or cytokines, such as Prf1 or Ifng, provide a powerful tool for exploring the role of GNLY, alone and in conjunction with other cytotoxic molecules and antiinfectious cytokines, in microbial defense. GNLY-Tg mice are much better at clearing intracellular bacterial (L. monocytogenes) and protozoan (T. cruzi, T. gondii) infection than WT mice and GNLY protection depends on PFN [*45,50]. In T. cruzi and T. gondii infections, lethal challenges in WT mice were well tolerated in GNLY-Tg mice, many of which cleared the infection. So far animal studies have been limited to these 3 infections. It is unclear whether this immune defense is physiologically important in vivo in other infections, including cell-free bacteria (given the high concentration of GNLY needed to kill bacteria ex vivo), fungi and malaria. It is clear, however, that mouse studies that have suggested a limited role for cytotoxicity in mouse models of infection need to be revisited to take into account the antimicrobial effects of GNLY.
Conclusions
GNLY is an exciting new player in microbial immune defense (Figure 4). Although studies beginning 20 years ago showed antimicrobial functions of GNLY on its own against cell-free bacteria, the high concentrations needed for GNLY to kill bacteria (and still higher concentrations to kill mammalian cells) raised questions about whether GNLY is protective in vivo. The recently discovered ability of GNLY at much lower nanomolar concentrations to deliver Gzms into microbes and kill them greatly expands its potential role in controlling infection. Although other GNLY-independent roles of T cells contribute to immune control of nonviral infections, such as killing of infected host cells and helper function for producing high affinity antibodies, the high susceptibility of AIDS and primary T cell immunodeficiency patients to bacterial, fungal and parasite infections suggests that GNLY-dependent immune defense is indeed important in humans. It is still unclear whether during some infectious or inflammatory conditions, such as in the synovial fluid of infected joints or skin abscesses or other localized infections, such as pneumonia, secreted GNLY and/or GNLY released during granule exocytosis together with Gzms and PFN, might help contain cell-free or invasive pathogens. Undoubtedly the local concentration of GNLY in the immune synapse during killer cell degranulation greatly surpasses the GNLY concentration in extracellular fluids, suggesting that in some cases GNLY delivered by killer cells to infected cells might directly kill microbes independently of PFN or Gzms. Recent studies suggest that NK cells bind to, degranulate and kill cell-free fungi when NK activating receptors bind to conserved fungal cell surface molecules, suggesting an unexpected role for killer lymphocytes in directly attacking fungi [62] and possibly some cell-free bacterial species.
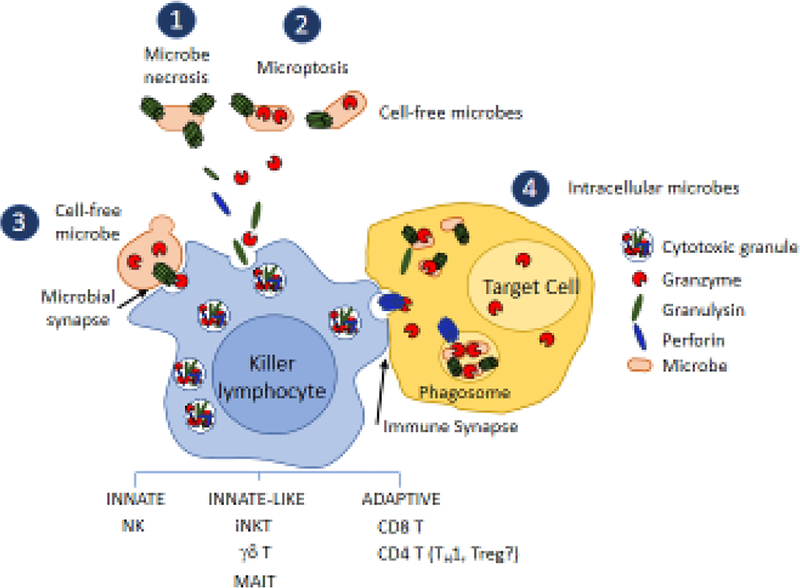
Fig. 4. Killer lymphocyte mechanisms to kill microbes.
Killer lymphocytes use GNLY to kill microbes by several mechanisms. Secreted GNLY on its own can damage bacterial membranes to cause loss of membrane integrity and necrotic cell death (1) or can deliver secreted GNLY into cell free microbes to cause microbial programmed cell death (microptosis) (2). Killer lymphocytes have been shown to directly form an immune synapse with cell free fungi utilizing the activating receptors NKp30 and NKp46, which bind to fungal glycans and trigger degranulation and fungal death (3). This mechanism of killing is not defined and presumably depends on GNLY and Gzms. Since some bacteria bear these glycans, cell-free bacteria may also be killed by this mechanism. Intracellular bacteria are also killed when killer lymphocytes recognize an infected cell, degranulate and kill the host cell (4). Intracellular microbes are usually killed before the host cell. Perforin delivers the Gzms and GNLY into the host cell, which can then kill microbes in the cytosol or within phagosomes. The relative importance of Gzm-dependent microptosis and Gzm- independent microbial necrosis is not known. It is still unclear whether secreted GNLY (with or without secreted Gzms) plays an important role in controlling infection in vivo and under what circumstances.
Since microptosis disrupts many vital microbial pathways and bacteria seem unable to easily develop resistance, one wonders whether GNLY might be used as a novel antibiotic either on its own or in combination with a Gzm-like protease. The relative selectivity of GNLY for damaging microbial versus mammalian membranes could mean a reasonable therapeutic index for an agent that could kill pathogens without destroying host cells. The conserved Gzm substrates might also point the way to new types of antibiotic drug targets worth investigating.
Highlights.
Granulysin is an antimicrobial peptide in CTL and NK cytotoxic granules
Granulysin permeabilizes cholestrerol-poor microbial membranes to deliver granzymes
Granulysin and granzymes activate microptosis, programmed cell death in microbes
GNLY-transgenic mice are protected from bacterial and parasite infections
Human GNLY+ antimicrobial CD8 T cells can be identified by NKG2C expression
Acknowledgments
We thank past and present members of the Lieberman lab who contributed to the research we have done on GNLY antimicrobial defense. The writing of this report was funded in part by NIH grants AI123265, AI116577, and AI131632 (J.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lieberman J: Cell-Mediated Cytotoxicity. In Fundamental Immunology, edn 7th. Edited by Paul WE: Wolters Kluwer/ Lippincott Williams and Wilkins; 2013:891–909. [Google Scholar]
- 2.Jongstra J, Schall TJ, Dyer BJ, Clayberger C, Jorgensen J, Davis MM, Krensky AM: The isolation and sequence of a novel gene from a human functional T cell line. The Journal of experimental medicine 1987, 165:601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munford RS, Sheppard PO, O’Hara PJ: Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. Journal of Lipid Research 1995, 36:1653–1663. [PubMed] [Google Scholar]
- 4.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH: CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol 2001, 167:2734–2742. [DOI] [PubMed] [Google Scholar]
- 5.Xing J, Wu F, Wang S, Krensky AM, Mody CH, Zheng C: Granulysin production and anticryptococcal activity is dependent upon a far upstream enhancer that binds STAT5 in human peripheral blood CD4+ T cells. J Immunol 2010, 185:5074–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda Y, Tamura T, Fukutomi Y, Mukai T, Kai M, Makino M: A lipopeptide facilitate induction of Mycobacterium leprae killing in host cells. PLoS Negl Trop Dis 2011, 5:e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veljkovic Vujaklija D, Dominovic M, Gulic T, Mahmutefendic H, Haller H, Saito S, Rukavina D: Granulysin expression and the interplay of granulysin and perforin at the maternal-fetal interface. J Reprod Immunol 2013, 97:186–196. [DOI] [PubMed] [Google Scholar]
- 8.Furudate S, Fujimura T, Kambayashi Y, Aiba S: Granulysin-Bearing Cells in the Skin Lesions of Acute Graft-versus-Host Disease: Possible Mechanisms for Hypohidrosis in Graft-versus-Host Disease. Case Rep Dermatol 2013, 5:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammar M, Mokni M, Boubaker S, El Gaied A, Ben Osman A, Louzir H: Involvement of granzyme B and granulysin in the cytotoxic response in lichen planus. J Cutan Pathol 2008, 35:630–634. [DOI] [PubMed] [Google Scholar]
- 10.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, et al. : Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008, 14:1343–1350. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Yoshioka N, Abe R, Murata J, Hoshina D, Mae H, Shimizu H: Rapid immunochromatographic test for serum granulysin is useful for the prediction of Stevens-Johnson syndrome and toxic epidermal necrolysis. J Am Acad Dermatol 2011, 65:65–68. [DOI] [PubMed] [Google Scholar]
- 12.Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, Hanson DA, Kluck RM, Hitoshi Y, Johnson DE, et al. : A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol 2001, 167:350–356. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J: Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitabut N, Mahasirimongkol S, Yanai H, Ridruechai C, Sakurada S, Dhepakson P, Kantipong P, Piyaworawong S, Moolphate S, Hansudewechakul C, et al. : Decreased plasma granulysin and increased interferon-gamma concentrations in patients with newly diagnosed and relapsed tuberculosis. Microbiol Immunol 2011, 55:565–573. [DOI] [PubMed] [Google Scholar]
- *15.Balin SJ, Pellegrini M, Klechevsky E, Won ST, Weiss DI, Choi AW, Hakimian J, Lu J, Ochoa MT, Bloom BR, et al. : Human antimicrobial cytotoxic T lymphocytes, defined by NK receptors and antimicrobial proteins, kill intracellular bacteria. Sci Immunol 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified the circulating human population of antimicrobial CD8 T cells in leprosy as triple positive for GNLY, GzmB and PFN that also coexpress the activating NK receptor NKG2C, which could be used to isolate them.
- 16.Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polanski K, Goncalves A, et al. : Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suda G, Yamamoto Y, Nagasaka A, Furuya K, Kudo M, Yoshimichi C, Tsukuda Y, Tsunematsu S, Sato F, Terasita K, et al. : Serum granulysin levels as a predictor of serious telaprevir-induced dermatological reactions. Hepatol Res 2014, 45:837–845. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Chen SA, Wu X, Zhu Q, Luo X: Overexpression of cytotoxic proteins correlates with liver function impairment in patients with drug reaction with eosinophilia and systemic symptoms (DRESS). Eur J Dermatol 2018, 28:13–25. [DOI] [PubMed] [Google Scholar]
- 19.Ono S, Otsuka A, Yamamoto Y, Kataoka TR, Koyanagi I, Miyachi Y, Kabashima K: Serum granulysin as a possible key marker of the activity of alopecia areata. J Dermatol Sci 2014, 73:74–79. [DOI] [PubMed] [Google Scholar]
- 20.Ochoa MT, Stenger S, Sieling PA, Thoma-Uszynski S, Sabet S, Cho S, Krensky AM, Rollinghoff M, Nunes Sarno E, Burdick AE, et al. : T-cell release of granulysin contributes to host defense in leprosy. Nat Med 2001, 7:174–179. [DOI] [PubMed] [Google Scholar]
- 21.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al. : An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998, 282:121–125. [DOI] [PubMed] [Google Scholar]
- 22.Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, Clayberger C, Krensky AM, Leippe M, Bloom BR, Ganz T, et al. : Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol 2000, 165:7102–7108. [DOI] [PubMed] [Google Scholar]
- 23.Barman H, Walch M, Latinovic-Golic S, Dumrese C, Dolder M, Groscurth P, Ziegler U: Cholesterol in negatively charged lipid bilayers modulates the effect of the antimicrobial protein granulysin. J Membr Biol 2006, 212:29–39. [DOI] [PubMed] [Google Scholar]
- 24.Walch M, Eppler E, Dumrese C, Barman H, Groscurth P, Ziegler U: Uptake of granulysin via lipid rafts leads to lysis of intracellular Listeria innocua. Journal of Immunology 2005, 174:4220–4227. [DOI] [PubMed] [Google Scholar]
- 25.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, Goping IS, Bleackley RC, Kirchhausen T, Lieberman J: Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nature immunology 2011, 12:770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walch M, Latinovic-Golic S, Velic A, Sundstrom H, Dumrese C, Wagner CA, Groscurth P, Ziegler U: Perforin enhances the granulysin-induced lysis of Listeria innocua in human dendritic cells. BMC immunology 2007, 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, et al. : Differential effects of cytolytic T cell subsets on intracellular infection. Science 1997, 276:1684–1687. [DOI] [PubMed] [Google Scholar]
- 28.Cho S, Mehra V, Thoma-Uszynski S, Stenger S, Serbina N, Mazzaccaro RJ, Flynn JL, Barnes PF, Southwood S, Celis E, et al. : Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci U S A 2000, 97:12210–12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabelitz D, Bender A, Schondelmaier S, Schoel B, Kaufmann SH: A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med 1990, 171:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Krensky AM, Bonneville M, Peyrat MA, Caccamo N, Sireci G, Salerno A: Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis 2001, 184:1082–1085. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Morita CT: Sensor Function for Butyrophilin 3A1 in Prenyl Pyrophosphate Stimulation of Human Vgamma2Vdelta2 T Cells. J Immunol 2015, 195:4583–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa G, Loizon S, Guenot M, Mocan I, Halary F, de Saint-Basile G, Pitard V, Dechanet-Merville J, Moreau JF, Troye-Blomberg M, et al. : Control of Plasmodium falciparum erythrocytic cycle: gammadelta T cells target the red blood cell-invasive merozoites. Blood 2011, 118:6952–6962. [DOI] [PubMed] [Google Scholar]
- 33.Farouk SE, Mincheva-Nilsson L, Krensky AM, Dieli F, Troye-Blomberg M: Gamma delta T cells inhibit in vitro growth of the asexual blood stages of Plasmodium falciparum by a granule exocytosis-dependent cytotoxic pathway that requires granulysin. European journal of immunology 2004, 34:2248–2256. [DOI] [PubMed] [Google Scholar]
- 34.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, et al. : Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013, 341:1359–1365. [DOI] [PubMed] [Google Scholar]
- 35.Ma LL, Spurrell JC, Wang JF, Neely GG, Epelman S, Krensky AM, Mody CH: CD8 T cell-mediated killing of Cryptococcus neoformans requires granulysin and is dependent on CD4 T cells and IL-15. J Immunol 2002, 169:5787–5795. [DOI] [PubMed] [Google Scholar]
- 36.Ma LL, Wang CL, Neely GG, Epelman S, Krensky AM, Mody CH: NK cells use perforin rather than granulysin for anticryptococcal activity. J Immunol 2004, 173:3357–3365. [DOI] [PubMed] [Google Scholar]
- 37.Oykhman P, Mody CH: Direct microbicidal activity of cytotoxic T-lymphocytes. J Biomed Biotechnol 2010, 2010:249482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy JW, Hidore MR, Wong SC: Direct interactions of human lymphocytes with the yeast-like organism, Cryptococcus neoformans. J Clin Invest 1993, 91:1553–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng CF, Ma LL, Jones GJ, Gill MJ, Krensky AM, Kubes P, Mody CH: Cytotoxic CD4+ T cells use granulysin to kill Cryptococcus neoformans, and activation of this pathway is defective in HIV patients. Blood 2007, 109:2049–2057. [DOI] [PubMed] [Google Scholar]
- *40.Vitenshtein A, Charpak-Amikam Y, Yamin R, Bauman Y, Isaacson B, Stein N, Berhani O, Dassa L, Gamliel M, Gur C, et al. : NK Cell Recognition of Candida glabrata through Binding of NKp46 and NCR1 to Fungal Ligands Epa1, Epa6, and Epa7. Cell Host Microbe 2016, 20:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that the activating NK receptor NKp46 binds to glycans on a fungal species to trigger degranulation and death.
- 41.Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, Xiang RF, Oykhman P, Huston SM, Islam A, et al. : The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe 2013, 14:387–397. [DOI] [PubMed] [Google Scholar]
- 42.Ogbomo H, Mody CH: Granule-Dependent Natural Killer Cell Cytotoxicity to Fungal Pathogens. Front Immunol 2016, 7:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Li SS, Ogbomo H, Mansour MK, Xiang RF, Szabo L, Munro F, Mukherjee P, Mariuzza RA, Amrein M, Vyas JM, et al. : Identification of the fungal ligand triggering cytotoxic PRR-mediated NK cell killing of Cryptococcus and Candida. Nat Commun 2018, 9:751. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a fungal glucan as the ligand for the human NK activating receptor NKp30 (a pseudogene in mice) for direct killing of cell-free fungi.
- 44.Huang LP, Lyu SC, Clayberger C, Krensky AM: Granulysin-mediated tumor rejection in transgenic mice. Journal of immunology 2007, 178:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Dotiwala F, Mulik S, Polidoro RB, Ansara JA, Burleigh BA, Walch M, Gazzinelli RT, Lieberman J: Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat Med 2016, 22:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that killer lymphocytes use GNLY, PFN and Gzms to induce oxidative damage that causes rapid programmed cell death in 3 protozoan parasites and that mice expressing the GNLY transgene survive infections that are lethal to widl-type mice.
- 46.van Meer G, Voelker DR, Feigenson GW: Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 2008, 9:112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maxfield FR, van Meer G: Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol 2010, 22:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H, Zhong C, Shi L, Guo Y, Fan Z: Granulysin induces cathepsin B release from lysosomes of target tumor cells to attack mitochondria through processing of bid leading to Necroptosis. J Immunol 2009, 182:6993–7000. [DOI] [PubMed] [Google Scholar]
- 49.Thiery J, Keefe D, Saffarian S, Martinvalet D, Walch M, Boucrot E, Kirchhausen T, Lieberman J: Perforin activates clathrin- and dynamin-dependent endocytosis, which is required for plasma membrane repair and delivery of granzyme B for granzyme-mediated apoptosis. Blood 2010, 115:1582–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, Krensky AM, Martinvalet D, Lieberman J: Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 2014, 157:1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **51.Dotiwala F, Sen Santara S, Binker-Cosen AA, Li B, Chandrasekaran S, Lieberman J:Granzyme B Disrupts Central Metabolism and Protein Synthesis in Bacteria to Promote an Immune Cell Death Program. Cell 2017, 171:1125–1137 e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes how killer cells cause microptosis, programmed cell death in bacteria, by cleaving enzymes that control protein synthesis and central metabolism.
- 52.Martinvalet D, Zhu P, Lieberman J: Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity 2005, 22:355–370. [DOI] [PubMed] [Google Scholar]
- 53.Martinvalet D, Dykxhoorn DM, Ferrini R, Lieberman J: Granzyme A cleaves a mitochondrial complex I protein to initiate caspase-independent cell death. Cell 2008, 133:681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacquemin G, Margiotta D, Kasahara A, Bassoy EY, Walch M, Thiery J, Lieberman J, Martinvalet D: Granzyme B-induced mitochondrial ROS are required for apoptosis. Cell Death Differ 2015, 22:862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Junqueira C, Barbosa CRR, Costa PAC, Teixeira-Carvalho A, Castro G, Sen Santara S, Barbosa RP, Dotiwala F, Pereira DB, Antonelli LR, et al. : Cytotoxic CD8(+) T cells recognize and kill Plasmodium vivax-infected reticulocytes. Nat Med 2018, 24:1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper uncovered an unexpected immune defense against blood-stage P. vivax malaria in which CD8 T cells recognize MHC induced by parasite infection on infected reticulocytes, lyse the red blood cell and directly kill the parasite, preventing it from spreading to infect new blood cells.
- 56.Thuong PH, Tam DB, Sakurada S, Hang NT, Hijikata M, Hong LT, Ngoc PT, Anh PT, Cuong VC, Matsushita I, et al. : Circulating granulysin levels in healthcare workers and latent tuberculosis infection estimated using interferon-gamma release assays. BMC Infect Dis 2016, 16:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pitabut N, Sakurada S, Tanaka T, Ridruechai C, Tanuma J, Aoki T, Kantipong P, Piyaworawong S, Kobayashi N, Dhepakson P, et al. : Potential function of granulysin, other related effector molecules and lymphocyte subsets in patients with TB and HIV/TB coinfection. Int J Med Sci 2013, 10:1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hogg AE, Bowick GC, Herzog NK, Cloyd MW, Endsley JJ: Induction of granulysin in CD8+ T cells by IL-21 and IL-15 is suppressed by human immunodeficiency virus-1. Journal of leukocyte biology 2009, 86:1191–1203. [DOI] [PubMed] [Google Scholar]
- 59.Andersson J, Samarina A, Fink J, Rahman S, Grundstrom S: Impaired expression of perforin and granulysin in CD8+ T cells at the site of infection in human chronic pulmonary tuberculosis. Infect Immun 2007, 75:5210–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kishi A, Takamori Y, Ogawa K, Takano S, Tomita S, Tanigawa M, Niman M, Kishida T, Fujita S: Differential expression of granulysin and perforin by NK cells in cancer patients and correlation of impaired granulysin expression with progression of cancer. Cancer Immunol Immunother 2002, 50:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. : Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005, 353:2654–2666. [DOI] [PubMed] [Google Scholar]
- 62.Ogbomo H, Timm-McCann M, Barnes T, Xiang RF, Jamil K, Ganguly A, Stack D, Huston SM, Li SS, Colarusso P, et al. : Granule-Dependent NK Cell Killing of Cryptococcus Requires Kinesin to Reposition the Cytolytic Machinery for Directed Cytotoxicity. Cell Rep 2018, 24:3017–3032. [DOI] [PubMed] [Google Scholar]