Figure 3. MPO-dependent events in human neutrophil phagosomes.
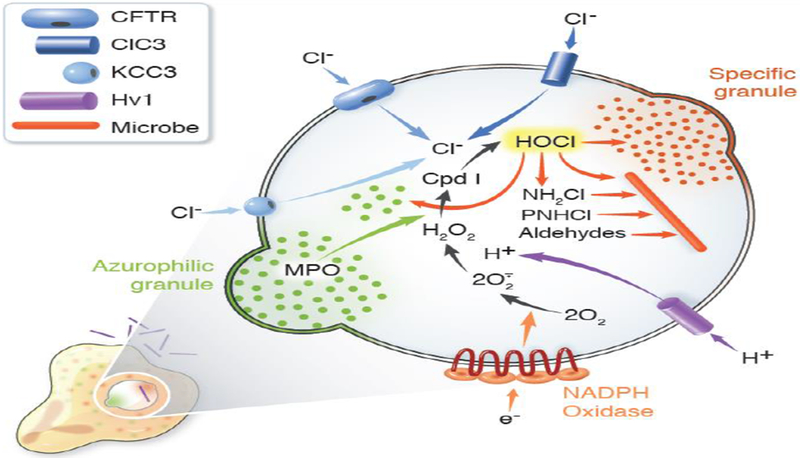
During phagocytosis, human neutrophils assemble and activate the NADPH oxidase and recruit granules to fuse with nascent phagosomes. Electrons transferred into the phagosome by the NADPH oxidase reduce molecular oxygen to superoxide anion (O2•−) and H2O2. The voltage-gated proton channel Hv1 delivers protons into the phagosome to compensate the charge generated by electron transfer from cytoplasm and thus sustain oxidase activity. The cystic fibrosis transmembrane conductance regulator (CFTR), with some contribution from chloride channel 3 (ClC3) and the potassium-chloride cotransporter (KCC3), provides chloride (Cl−) from the neutrophil cytoplasm. MPO, delivered by fusion of azurophilic granules, and H2O2, generated by the oxidase, react to yield Compound I (Cpd I) that in turn catalyzes the oxidation of Cl− to produce hypochlorous acid (HOCl) or bleach. HOCl reacts with targets both from host and microbe to generate a variety of reactive products, including monochloramines (NH2Cl) and protein chloramines (PNHCl), which can then decompose to form aldehydes. Collectively, the granule proteins, acting in both their native and oxidant-modified forms, along with HOCl and its derivatives act synergistically to attack microbial targets. Figure from [40] and used with permission.
