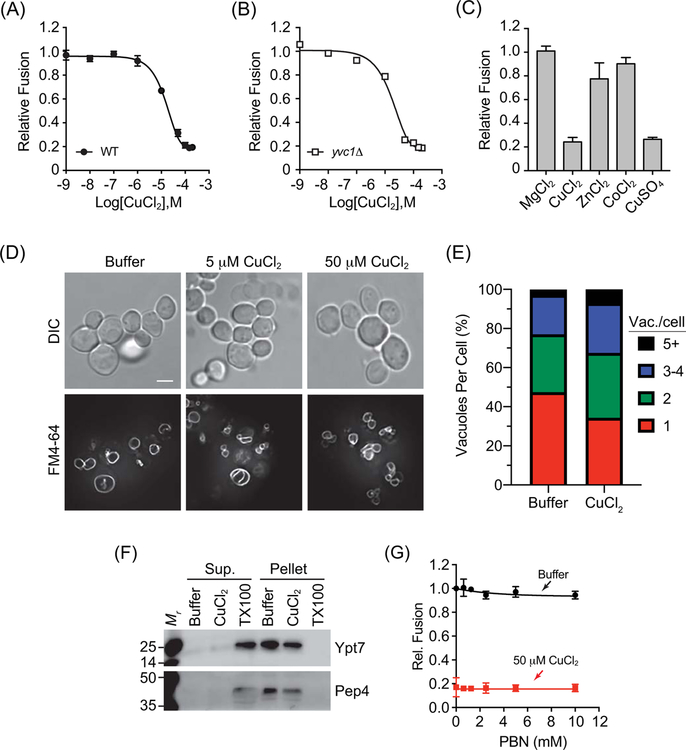
Figure 1. CuCl2 inhibits vacuole homotypic fusion.
Vacuoles isolated from wild type (BJ3505 and DKY6281) (A) or yvc1Δ (RFY74–75) (B) fusion reporter strains were incubated with buffer alone or a concentration curve of CuCl2. (C) Wild type vacuoles were incubated with 100 μM MgCl2, ZnCl2, CoCl2, CuCl2, or CuSO4. Fusion reactions were incubated for 90 min at 27°C. After incubation membranes were solubilized and incubated with p-nitrophenyl phosphate to measure Pho8 activity. p-nitrophenolate was measured at OD400. Fusion values were normalized to the untreated control set to 1. (D) BJ3505 cells were grown to log phase and treated with CuCl2. Vacuoles were visualized by staining with FM4–64 and cells were visualized using DIC. (E) Quantitation of vacuole fragmentation of cells treated with 0 μM or 50 μM CuCl2 as in panel D. n=392, untreated cells, n=616, 50 μM CuCl2 treated cells. (F) DKY6281 vacuoles were incubated with 100 μM CuCl2, 0.1% TX-100 or buffer for 90 min at 27°C. After incubation, the soluble and membrane fractions were separated by centrifugation (16,000 x g, 10 min, 4°C), mixed with SDS-loading buffer and resolved by SDS-PAGE. The soluble luminal protease Pep4 and the membrane anchored Ypt7 were probed for by immunoblotting. (G) Wild type vacuoles were treated with or without CuCl2 in the presence of a concentration curve of PBN to eliminate oxygen radicals. Error bars are S.E.M. (n=3). Scale bar = 4 μm.