Abstract
Background:
The optimal approach at treating infected biliary stents at the time of cholangitis remains unknown. This study aims to compare the efficacy of stent exchange versus stent sweeping/stent-in-stent approaches at treating cholangitis.
Methods:
The study was a retrospective cohort study. Patients with biliary stents and cholangitis were included. Outcomes were rate of recurrent cholangitis and time to recurrent cholangitis in those whose stents were left in place (stent sweeping and stent-in-stent) compared to those whose stents were removed (stent exchange). Primary analysis included patients with metal biliary stents only. Secondary analysis included those with metal and plastic biliary stents.
Results:
A total of 182 patients (age 64±12;89F) with a metal biliary stent(s) at index cholangitis were included. Of these, 40 (22%) had stents removed, i.e. stent exchange. The remaining 142 (78%) did not have stent removal (97 with stent-in-stent and 45 with stent sweeping). Recurrent cholangitis occurred in 22.5% and 42.3% in the stent removal and non-removal groups, respectively (p=0.02). Stent removal remained a negative predictor of recurrent cholangitis after controlling for age, sex, history of diabetes and chemotherapy (OR=0.39, p=0.03). Median time from index cholangitis to recurrent cholangitis was shorter for patients whose stents were not removed compared to those whose stents were removed (182 versus 450 days, p=0.011). On Cox regression model, stent removal remained a negative predictor of recurrent cholangitis after controlling for age, sex, history of diabetes and chemotherapy (HR=0.41, p=0.01). The findings persisted in the secondary analysis including both metal and plastic biliary stents (303 patients).
Conclusion:
Biliary stent removal with stent exchange at the time of cholangitis appears to be more effective at preventing recurrent cholangitis than leaving an infected stent in the biliary system.
Keywords: Cholangitis, biliary stent, stent occlusion, biofilm, stent exchange, sweeping, stent-in-stent
INTRODUCTION
Cholangitis is a clinical syndrome characterized by abdominal pain, jaundice and fever [1–2]. Two factors are required for cholangitis to occur including (1) biliary obstruction and stasis and (2) bile infection. Common etiologies of bile obstruction and stasis are choledocholithiasis (28–70%), benign biliary stricture (5–28%) and stenosis caused by malignancy (10–57%) [3–7]. In addition, cholangitis is a common complication of biliary stent placement due to stent occlusion with a reported incidence of approximately 15% [8–9].
Management of cholangitis is performed according to the severity grade of the patient. According to the updated Tokyo Guidelines 2013, patients with grade II (moderate) and grade III (severe) acute cholangitis, should undergo early and urgent biliary drainage, respectively. While patients with grade I (mild) acute cholangitis may initially be treated with supportive care and antibiotics, biliary drainage should be considered in those who do not respond to medical management [10–11]. Endoscopic Retrograde Cholangiopancreatography (ERCP) remains the gold standard treatment option for biliary drainage. For patients with cholangitis secondary to choledocholithiasis, ERCP with biliary sphincterotomy and stone removal is the preferred method. However, best practice for endoscopic management of patients with biliary stenosis and cholangitis secondary to stent occlusion remains unknown. Available options vary and include mechanical cleaning with balloon sweeps, stent-in-stent and stent exchange.
This study aims to compare the outcomes of different endoscopic techniques at treating patients with cholangitis secondary to stent occlusion. Specifically, we hypothesize that the techniques that involve removal of all existing stents, such as stent exchange and stent removal, would lead to a lower rate of recurrent cholangitis and longer time to recurrent cholangitis compared to those that do not remove all existing stents, such as balloon sweeping and stent-in-stent technique. Given the scheduled stent exchange with plastic stents, which may affect the rate of and time to recurrent cholangitis, the primary analysis focuses only on patients with metal biliary stents at the time of index cholangitis. The secondary analysis is then conducted to include patients with all stent types to assess generalizability of our hypothesis.
METHODS
Study Design and Data Collection
The study was a retrospective observational study of patients who underwent ERCP for ascending cholangitis at a tertiary academic center from 2006 to 2016. Only patients who had at least one biliary stent at the time of cholangitis were included. Exclusion criteria were an absence of a biliary stent at the time of cholangitis and an absence of fevers, leukocytosis, bandemia or pus seen on ERCP despite clinical suspicion for cholangitis. Collected data included age, sex, history of diabetes (yes or no), prior history of chemotherapy (yes or no), type of biliary stent (fully covered self-expandable metal stent (SEMS), uncovered or partially covered SEMS and plastic stent), indication for stent placement, date of biliary stent placement, date of index cholangitis, date of recurrent cholangitis (if any) and date of last follow-up. Additionally, details of endoscopic intervention during the index cholangitis were gathered. The primary analysis included only patients with metal biliary stent(s), while the secondary analysis included patients with either metal or plastic biliary stent(s).
Definitions:
Index cholangitis was defined as the first cholangitis episode that occurred when there was at least one biliary stent present. Recurrent cholangitis was defined as the subsequent episode(s) of cholangitis after the index episode. Stent removal referred to removal of all biliary stent(s) at the time of index cholangitis with placement of a new biliary stent, i.e. stent exchange. Of note, for patients with benign stricture, only those who required stent exchange due to persistent stenosis were included. Stent non-removal was when none or some but not all biliary stents were removed at the time of index cholangitis. This included balloon sweeping alone and a stent-in-stent technique.
Part 1—Rate of Recurrent Cholangitis:
This section assessed the rate of recurrent cholangitis in patients whose stents were removed compared to those whose stents were not removed at the time of index cholangitis. Possible confounders included age, sex, history of diabetes at the time of index cholangitis, and history of chemotherapy within one year prior to the index cholangitis, and were adjusted for using a logistic regression analysis.
Part 2—Time to Recurrent Cholangitis:
This section assessed time to recurrent cholangitis in patients whose stents were removed compared to those whose stents were not removed at the time of index cholangitis. Possible confounders included age, sex, history of diabetes at the time of index cholangitis, and history of chemotherapy within one year prior to the index cholangitis, and were adjusted for using a Cox regression analysis.
Statistical Analysis
All continuous variables were expressed as mean ± standard deviation (SD), and skewed variables were expressed as median and range. Categorical variables were expressed as proportions (%). Student’s t-test was used to compare continuous variables and Pearson’s chi-squared test was used for comparison of categorical variables. Univariable and multivariable logistic regression analyses were performed to assess an association between possible risk factors and recurrent cholangitis (stent removal at the time of index cholangitis, age, sex, history of diabetes at the time of index cholangitis and history of chemotherapy within one year prior to the index cholangitis). Given collinearity between stent removal and stent type, only stent removal was included in the model. Time to recurrent cholangitis of each study group was reported using a median. A time-to-event analysis with Kaplan Meier curves and a log rank test were used to compare time to recurrent cholangitis between study groups. A Cox proportional hazards regression analysis was used to adjust for potential confounders of incident recurrent cholangitis (stent removal at the time of index cholangitis, age, sex, history of diabetes at the time of index cholangitis and history of chemotherapy within one year prior to the index cholangitis). All tests were performed two-sided and a significance level of P ≤ 0.05 was considered statistical significance. All statistical analyses were performed using SAS Software Version 9.4 (Cary, NC). The study was approved by the Institutional Review Board (IRB).
RESULTS
Part 1: Rate of Recurrent Cholangitis
Primary Analysis: Metal Stents Only
A total of 182 patients who had at least one biliary metal stent at the time of index cholangitis were included. Of these, 97 (53%), 65 (36%), 8 (4%) and 12 (7%) patients had uncovered SEMS, fully covered SEMS, fully covered within uncovered SEMS and unspecified types of SEMS, respectively. Baseline characteristics are shown in Table 1. Indications for biliary metal stent placement were pancreatic adenocarcinoma (46%), nonbiliary neoplasms such as metastatic disease (34%), cholangiocarcinoma (13%), benign stricture (3%), ampullary neoplasm (2%) and others (2%).
Table 1.
Baseline characteristics of patients who had at least one biliary metal stent at the time of index cholangitis. Data presented as mean ± S.D. or median [min,max] or number of events (%).
| Characteristics | Entire Cohort (N = 182) | Stent Removed (N =40) | Stent not Removed (N =142) | P-value |
|---|---|---|---|---|
| Age (years) | 64 ± 12 | 66 ± 13 | 64 ± 12 | 0.36 |
| Sex (female) | 89 (49) | 15 (38) | 74 (52) | 0.10 |
| History of diabetes | 54 (30) | 12 (30) | 42 (30) | 0.96 |
| History of chemotherapy | 128 (70) | 19 (48) | 109 (77) | 0.003 |
| Time interval between stent placement and index cholangitis (months) | 125 [1,990] | 159 [4,695] | 113 [1,990] | -- |
Of the 182 patients, 40 (22%) had stent exchange. The remaining 142 (78%) did not have all stents removed but instead had stent within stent placement after sweeping (97) or stent sweeping alone (45).
Compared to those whose stents were not removed at the time of index cholangitis, those whose stents were removed experienced fewer episodes of recurrent cholangitis (22.5% in the removal group versus 42.3% in the non-removal group, p = 0.02).
Univariable logistic regression analysis showed that stent removal was associated with a decreased risk of recurrent cholangitis (p = 0.023), while age, sex, history of diabetes and history of chemotherapy were not (p = 0.17, 0.94, 0.06 and 0.62, respectively). Multivariable regression analysis showed that stent removal remained a significant negative predictor of recurrent cholangitis after controlling for age, sex, history of diabetes and history of chemotherapy (OR = 0.39, p = 0.03) (Table 2).
Table 2.
Multivariable logistic regression of variables associated with recurrent cholangitis in patients who had at least one biliary metal stent at the time of index cholangitis.
| Variables | β ± S.E. | Odds Ratio [95% CI] | P-value |
|---|---|---|---|
| Age | −0.01 ± 0.01 | 0.99 [0.97,1.02] | 0.55 |
| Sex | 0.06 ± 0.32 | 1.06 [0.57,1.99] | 0.85 |
| Stent Removal | −0.94 ± 0.43 | 0.39 [0.17,0.92] | 0.03 |
| History of diabetes | 0.66 ± 0.34 | 1.93 [0.99,3.78] | 0.06 |
| History of chemotherapy | −0.005 ± 0.37 | 1.00 [0.49,2.04] | 0.99 |
Secondary Analysis: Metal Stents and Plastic Stents
A total of 303 patients who had at least one biliary metal or plastic stent at the time of index cholangitis were included in the secondary analysis. Of these, 183 (60%), 111 (37%) and 9 (3%) patients had metal stents, plastic stents and plastic within metal stents, respectively. Baseline characteristics and indications for biliary stent placement are shown in Table 3. Indications for biliary stent placement were pancreatic adenocarcinoma (39%), nonbiliary neoplasms such as metastatic disease (30%), cholangiocarcinoma (17%), benign stricture (7%), stones (3%), ampullary neoplasm (2%) and others (2%).
Table 3.
Baseline characteristics of patients who had at least one biliary metal or plastic stent at the time of index cholangitis. Data presented as mean ± S.D. or number of events (%).
| Characteristics | Entire Cohort (N = 303) | Stent Removed (N =149) | Stent not Removed (N =154) | P-value |
|---|---|---|---|---|
| Age (years) | 63 ± 13 | 63 ± 14 | 63 ± 12 | 1.00 |
| Sex (female) | 137 (45) | 61 (40) | 76 (50) | 0.08 |
| History of diabetes | 106 (35) | 53 (35) | 53 (35) | 1.00 |
| History of chemotherapy | 199 (65) | 87 (57) | 112 (74) | 0.003 |
| Time interval between stent placement and index cholangitis (months) | 81 [1,980] | 48 [3,553] | 132 [1,980] | -- |
Of the 303 patients, 149 (49%) had stent exchange. The remaining 154 (51%) did not have the stents removed but instead had stent within stent placement after sweeping (109) or stent sweeping alone (45).
Compared to those whose stents were not removed at the time of index cholangitis, those whose stents were removed experienced fewer episodes of recurrent cholangitis (30.2% in the removal group versus 42.2% in the non-removal group, p = 0.03).
Univariable logistic regression analysis showed that stent removal was associated with a decreased risk of recurrent cholangitis (p = 0.03), while age, sex, history of diabetes and history of chemotherapy were not (p = 0.44, 0.70, 0.71 and 0.20, respectively). Multivariable regression analysis showed that stent removal remained a significant negative predictor of recurrent cholangitis after controlling for age, sex, history of diabetes and history of chemotherapy (OR = 0.61, p = 0.048) (Table 4).
Table 4.
Multivariable logistic regression of variables associated with recurrent cholangitis in patients who had at least one biliary metal or plastic stent at the time of index cholangitis.
| Variables | β ± S.E. | Odds Ratio [95% CI] | P-value |
|---|---|---|---|
| Age | −0.01 ± 0.01 | 0.99 [0.97,1.01] | 0.17 |
| Sex | −0.03 ± 0.25 | 0.97 [0.60,1.57] | 0.89 |
| Stent Removal | −0.49 ± 0.25 | 0.61 [0.38,0.995] | 0.048 |
| History of diabetes | −0.04 ± 0.26 | 0.96 [0.58,1.59] | 0.87 |
| History of chemotherapy | 0.24 ± 0.26 | 1.27 [0.76,2.13] | 0.37 |
Part 2: Time to Recurrent Cholangitis and Cox Proportional Hazards Model
Primary Analysis: Metal Stents Only
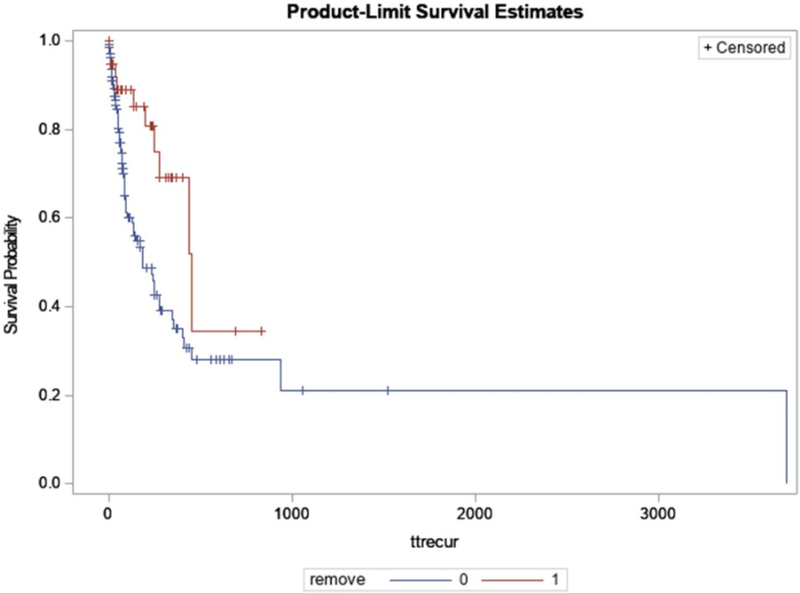
Median time from the index cholangitis to the first episode of recurrent cholangitis was shorter for patients whose stents were not removed at time of index cholangitis compared to those whose stents were removed (182 days in the non-removal group versus 450 days in the removal group, p = 0.011) (Figure 1).
Figure 1.
Kaplan-Meier curve for recurrent cholangitis-free survival in patients with biliary metal stent(s) stratified by stent removal at the time of index cholangitis
On a Cox regression model, stent removal was the only significant predictor of recurrent cholangitis with an adjusted hazard ratio of 0.41 (p = 0.01) after controlling for age, sex, diabetic status and history of prior chemotherapy (Table 5).
Table 5.
Cox regression analysis of variables associated with recurrent cholangitis in patients who had at least one biliary metal stent at the time of index cholangitis.
| Variables | β-coefficient | Adjusted Hazard Ratio | P-value |
|---|---|---|---|
| Age | −0.004 ± 0.01 | 1.00 [0.98,1.02] | 0.73 |
| Sex | 0.36 ± 0.27 | 1.43 [0.85,2.41] | 0.18 |
| Stent removal | −0.90 ± 0.35 | 0.41 [0.20,0.81] | 0.01 |
| Diabetes Status | −0.04 ± 0.26 | 0.96 [0.58,1.59] | 0.89 |
| Chemotherapy | 0.03 ± 0.28 | 1.03 [0.60,1.79] | 0.91 |
Secondary Analysis: Metal Stents and Plastic Stents
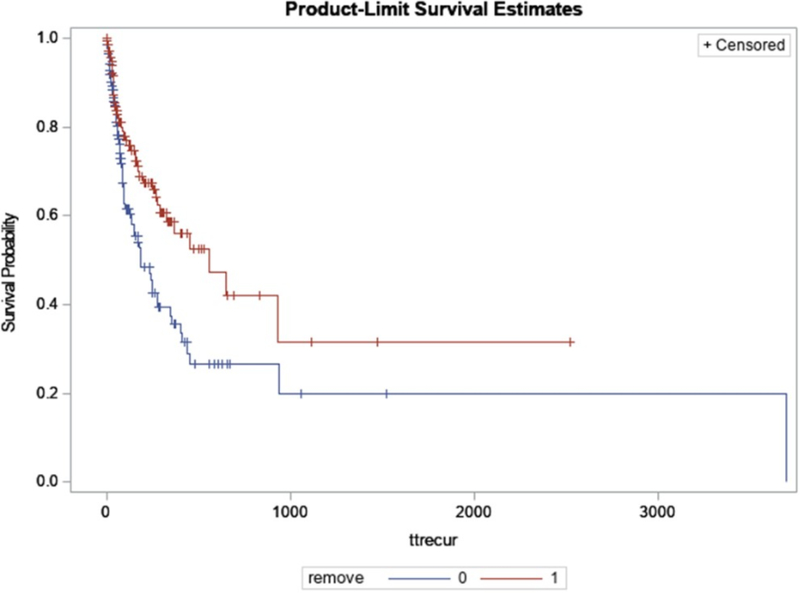
Median time from the index cholangitis to the first episode of recurrent cholangitis was shorter for patients whose stents were not removed at time of index cholangitis compared to those whose stents were removed (182 days in the non-removal group versus 559 days in the removal group, p = 0.005) (Figure 2).
Figure 2.
Kaplan-Meier curve for recurrent cholangitis-free survival in patients with biliary metal or plastic stent(s) stratified by stent removal at the time of index cholangitis
On a Cox regression model, stent removal was the only significant predictor of recurrent cholangitis with an adjusted hazard ratio of 0.58 (p = 0.01) after controlling for age, sex, diabetic status and history of prior chemotherapy (Table 6).
Table 6.
Cox regression analysis of variables associated with recurrent cholangitis in patients who had at least one biliary metal or plastic stent at the time of index cholangitis.
| Variables | β-coefficient | Adjusted Hazard Ratio | P-value |
|---|---|---|---|
| Age | −0.01 ± 0.01 | 0.99 [0.98,1.01] | 0.25 |
| Sex | 0.19 ± 0.20 | 1.21 [0.81,1.79] | 0.36 |
| Stent removal | −0.55 ± 0.20 | 0.58 [0.39,0.86] | 0.01 |
| Diabetes Status | −0.27 ± 0.21 | 0.77 [0.51,1.15] | 0.20 |
| Chemotherapy | 0.24 ± 0.21 | 1.27 [0.84,1.92] | 0.27 |
DISCUSSION
This study demonstrates that removal of all existing biliary stents at the time of cholangitis is associated with a decreased risk of recurrent cholangitis. Additionally, stent removal appears to be associated with a longer time duration between the index cholangitis and the episode of recurrent cholangitis.
Management of acute cholangitis as a result of occluded biliary stent(s) remains controversial. To date, endoscopic treatment options include mechanical cleaning with a balloon, a second stent insertion as stent-in-stent and stent exchange. Comparison studies on different endoscopic techniques for the treatment of occluded biliary stents remain relatively limited. Specifically, previous studies focused on comparing the efficacy of different types of stents to be inserted within the existing stent for the stent-in-stent technique. Four studies demonstrated that placement of a metal stent within the occluded stent was associated with increased patency compared to placement of a plastic stent. One study showed the highest patency rate with a fully covered metal stent placed within the occluded stent [12–15]. Nevertheless, two other studies showed no difference in adequacy of biliary drainage if a metal or plastic stent was placed within the occluded metal stent [16–17].
As far as we know, this is the first study to compare the different endoscopic techniques at treating infection due to occluded biliary stents. The study showed that removal of all existing stents with placement of a new stent(s) led to decreased risk of and a longer duration between episodes of cholangitis. These findings persisted after controlling for potential confounders for recurrent cholangitis including age, sex and an immunocompromised state such as history of diabetes and previous chemotherapy. As a result, when selecting a biliary stent, those that are not designed for removal, such as uncovered metal stents, should be used with caution.
As the focus of this study was on the impact of stent removal, and not stent type, in the management of cholangitis, plastic stents were also included in the secondary analysis. Plastic stents were found to be universally removed for cholangitis, and similarly resulted in lower rates of recurrent cholangitis compared to metal stent cleaning and the stent-in-stent technique.
Despite the existing literature comparing different types of biliary stents as summarized above, to date, no studies have assessed the effect of biliary stents on recurrent cholangitis following the index episode of cholangitis. Our study demonstrates that in order to decrease the risk of subsequent episodes of cholangitis, the ability to remove the infected stent is important. Specifically, regardless of the type of stent used, a practice of stent exchange rather than stent sweeping or stent-in-stent should be the preferred method for treating cholangitis due to a decreased risk and a longer duration between episodes of cholangitis.
Mechanisms of biliary stent obstruction are thought to be multifactorial. Bacterial biofilm, biliary sludge, bile viscosity, bacterial infection and duodenobiliary reflux of dietary fiber are thought to play a role [26–28]. Specifically, it has been proposed that the initial step of stent occlusion is bacterial colonization. Microorganisms isolated from occluded biliary stents include anaerobic and aerobic bacteria and fungi [29]. These microorganisms then excrete several types of protein, such as fibronectin, vitronectin, laminin, fibrin and collagen, which form a biofilm making the bacteria more adhesive. The biofilm on the inner surface of a stent is thought to make the inner surface irregular, which further facilitates the accumulation of sludge and debris [30–32]. It is possible that despite attempts to clean the occluded stent with balloon sweeping with or without placement of an additional stent, the original indwelling biliary stent is still coated with bacterial biofilm, causing an irregular inner surface of the stent which accelerates stent occlusion and recurrence of cholangitis.
Our study had a few limitations. First, since endoscopic techniques (stent removal versus non-removal) were covariates with the stent type, specific stent type could not be put into the regression models. To control for this, the primary analysis focused only on metal biliary stents, while the second analysis included both metal and plastic stents. Findings from our study remained the same with stent exchange being preferred to stent-in-stent or sweeping. In this study, a further subgroup analysis of different metal stent types, including fully-covered, partially covered and uncovered, was not performed due to a relatively small number of patients within each group leading to the analysis being underpowered. This analysis would be appropriate for a future multi-center study. Additionally, in this study, history of chemotherapy, which was previously demonstrated to increase the risk of recurrent cholangitis [33–34], was used as a potential confounder of recurrent cholangitis in the multivariable logistic and cox regression models. Given that history of malignancy and chemotherapy were covariates, history of malignancy was therefore unable to be put into the model. Furthermore, the secondary analysis included plastic stents, which are universally removed for cholangitis. As such, none of these stents were included in other arms leading to increased variability.
In conclusion, leaving an infected stent in the biliary system is associated with a higher rate of recurrent cholangitis compared to removal and replacement with a new stent. Unlike fully covered metal biliary stents, uncovered metal stents are not designed for removal and should be used with caution. Additionally, plastic stents are viable alternatives for hilar lesion where fully covered stents are not appropriate, provided that scheduled exchanges or laboratory surveillance is in place.
Acknowledgments
Study Support: None
Footnotes
AUTHOR DISCLOSURES
Drs. Pichamol Jirapinyo, Mohd AlSamman and Christopher Thompson have no conflicts of interest or financial ties to disclose.
REFERENCES
- 1.Charcot M. Comparison avec la fievre uroseptique. Lecons sur les maladies du foie des voies biliares et des reins. Paris: Bourneville et Sevestre; 1877. De la fievre hepatique symptomatique; pp. 176–85. [Google Scholar]
- 2.Reynolds BM, Dargan EL (August 1959). “Acute obstructive cholangitis; a distinct clinical syndrome”. Ann Surg. 150 (2): 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura Y, Takada T, Kawarada Y, Nimura Y, Hirata K, Sekimoto M, Yoshida M, Mayumi T, Wada K, Miura F, Yasuda H, Yamashita Y, Nagino M, Hirota M, Tanaka A, Tsuyuguchi T, Strasberg SM, Gadacz TR. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo Guidelines. J Hepatobiliary PANCREAT Surg. 2007;14(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gigot JF, Leese T, Dereme T, Coutinho J, Castaing D, Bismuth H. Acute cholangitis: multivariate analysis of risk factors. Ann Surg. 1989;209:435–8. doi: 10.1097/00000658-198904000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saharia PC, Cameron JL. Clinical management of acute cholangitis. Surg Gynecol Obstet. 1976;142:369–72. [PubMed] [Google Scholar]
- 6.Thompson JE Jr, Pitt HA, Doty JE, Coleman J, Irving C. Broad spectrum penicillin as an adequate therapy for acute cholangitis. Surg Gynecol Obstet. 1990;171:275–82. [PubMed] [Google Scholar]
- 7.Basoli A, Schietroma M, De Santis A, Colella A, Fiocca F, Speranza V. Acute cholangitis: diagnostic and therapeutic problems. Ital J Surg Sci. 1986;16:261–7. [PubMed] [Google Scholar]
- 8.Huibregtse K, Carr-Locke DL, Cremer M, et al. Biliary stent occlusion—a problem solved with self-expanding metal stents? European Wallstent Study Group. Endoscopy. 1992;24(5):391–4. [DOI] [PubMed] [Google Scholar]
- 9.Knyrim K, Wagner HJ, Pausch J, et al. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25(3):207–12. [DOI] [PubMed] [Google Scholar]
- 10.Kiriyama S, Takada T, Strasberg SM, et al. New diagnostic criteria and severity assessment of acute cholangitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19(5):548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura F, Takada T, Stransberg SM, et al. TG13 flowchart for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2013;20:47–54. [DOI] [PubMed] [Google Scholar]
- 12.Ridtitid W, Rerknimitr R, Janchai A, et al. Outcome of second interventions for occluded metallic stents in patients with malignant biliary obstruction. Surg Endosc. 2010;42:2216–20. [DOI] [PubMed] [Google Scholar]
- 13.Togawa O, Kawabe T, Isayama H, et al. Management of occluded uncovered metallic stents in patients with malignant distal biliary obstructions using covered metallic stents. J Clin Gastroenterol. 2008;42:546–9. [DOI] [PubMed] [Google Scholar]
- 14.Bueno JT, Gerdes H, Kurtz RC. Endoscopic management of occluded biliary Wallstents: a cancer center experience. Gastrointest Endosc. 2003;58:879–84. [DOI] [PubMed] [Google Scholar]
- 15.Rogant JN, Jain D, Siddiqui UD, et al. Analysis of endoscopic management of occluded metal biliary stents at a single tertiary care center. Gastrointest Endosc. 2008;68:676–82. [DOI] [PubMed] [Google Scholar]
- 16.Tham TC, Carr-Locke DL, Vandervoort J, et al. Management of occluded biliary Wallstents. Gut. 1998;42:703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon WJ, Ryu JK, Lee JW, et al. Endoscopic management of occluded metal biliary stents: metal versus 10F plastic stents. World J Gastroenterol. 2010;14:5347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullman E, Frozanpor F, Soderlund C, et al. Covered vs. uncovered self-expandable nitinol stents I the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915–23. [DOI] [PubMed] [Google Scholar]
- 19.Telford JJ, Carr-Locke DL, Baron TH, et al. A randomized controlled trial comparing uncovered and partially covered self-expandable metal stents in the palliation of distal malignant biliary obstruction. Gastrointest Endosc. 2010;72:907–14. [DOI] [PubMed] [Google Scholar]
- 20.Isayama H, Komatsu Y, Tsujino, et al. A prospective randomized study of covered vs. uncovered diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salleem A, Leggett CI, Murad MH, et al. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321–7. [DOI] [PubMed] [Google Scholar]
- 22.Wagner HJ, Knyrim K, Vakil N, et al. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction. A prospective and randomized trial. Endoscopy. 1993;25(3):213–8. [DOI] [PubMed] [Google Scholar]
- 23.Sangchan A, Kongkasame W, Pugkhem A, et al. Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc. 2012;76(1):93–9. [DOI] [PubMed] [Google Scholar]
- 24.Mukai T, Yasuda I, Nakashima M, et al. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013;20(2):214–22. [DOI] [PubMed] [Google Scholar]
- 25.Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28(4):593–607. [DOI] [PubMed] [Google Scholar]
- 26.Libby ED, Leung JW. Prevention of biliary stent clogging: a clinical review. Am J Gastroenterol. 1996;91:1301–1308. [PubMed] [Google Scholar]
- 27.Leung JW, Liu Y, Chan RC, et al. Early attachment of anaerobic bacteria may play an important role in biliary stent blockage. Gastrointest Endosc. 2000;52:725–729. [DOI] [PubMed] [Google Scholar]
- 28.van Berkel AM, van Marle J, Groen AK, et al. Mechanisms of biliary stent clogging: confocal laser scanning and scanning electron microscopy. Endoscopy. 2005;37:729–734. [DOI] [PubMed] [Google Scholar]
- 29.Donelli G, Guaglianone E, Di Rosa R, et al. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clin Med Res. 2007;5:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moesch C, Sautereau D, Cessot F, et al. Physicochemical and bacteriological analysis of the contents of occluded biliary endoprostheses. Hepatology. 1991;13:1142–1146. [PubMed] [Google Scholar]
- 31.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J BiomedMaterRes. 1998;43:338–348. [DOI] [PubMed] [Google Scholar]
- 32.Yu JL, Anderson R, Wang LQ. Fibronectin on the surface of biliary drain materials: a role in bacterial adherence. J Surg Res. 1995;59:596–600. [DOI] [PubMed] [Google Scholar]
- 33.Landau O, Kott I, Deutsch AA, et al. Multifactorial analysis of septic bile and septic complications in biliary surgery. World J Surg. 1992;16:962–964. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto J, Morizane C, Kondo S, et al. Incidence and risk factors for cholangitis during systemic chemotherapy among patients with advanced biliary tract cancer. Journal of Clinical Oncology. 2011;29:313. [Google Scholar]