Abstract
Objectives:
While multiple individual sleep measures (e.g., sleep duration, satisfaction) have been linked to a wide range of physical and mental health conditions, scant research has examined how individual sleep dimensions may act independently or additively to influence health. The current study investigates associations of five sleep dimensions (duration, satisfaction, efficiency, timing, and regularity), analyzed separately and simultaneously, with psychological distress, body mass index, and physical functioning among a low-income, predominantly African American population.
Design:
We constructed a composite Sleep Health (SH) score from the sum of scores, representing “good’ and “poor” ranges of five sleep measures (Range = 0 –5).
Setting:
Two low-income, predominantly African American neighborhoods in Pittsburgh.
Participants:
Participants included 738 community-dwelling adults (78% female and 98% Black).
Measurements:
Actigraphy-based measures of sleep duration, regularity, timing, and efficiency, and self-reported sleep satisfaction. Outcomes included self-reported psychological distress, physical functioning and measured BMI.
Results:
Each one-unit higher SH score was associated with 0.55-unit lower psychological distress score (range 0 to 24) and 2.23-unit higher physical functioning score. Participants with at least two, three, or four sleep dimensions in the “healthy” range, versus fewer, had lower psychological distress scores. Greater sleep satisfaction was associated with higher physical functioning, and longer sleep duration was associated with lower physical functioning. Neither the composite SH score nor any of the individual sleep dimensions were associated with BMI.
Conclusions:
Assessing multiple sleep dimensions may provide a more comprehensive understanding of associations of sleep with psychological distress than assessing any single sleep dimension. Although no sleep measures were related to BMI in the current sample, analyses should be replicated in other samples to determine generalizability.
Keywords: actigraphy, sleep health score, psychological distress, physical functioning
Various sleep dimensions, including poor sleep quality, extreme sleep durations, and irregular sleep timing, are demonstrated risk factors for a host of adverse physical and mental health outcomes (1–4). However, most studies to date linking sleep to health have focused on sleep disorders or sleep duration, while fewer have considered other sleep dimensions, such as sleep efficiency, timing, and regularity (2). Moreover, multiple sleep dimensions occur simultaneously in all individuals, regardless of the presence of clinical sleep disorders, and the combination of multiple sleep dimensions may interact to influence health in a manner that is qualitatively different than any single sleep dimension. A more holistic view of sleep health, with better understanding of how various sleep dimensions may be complementary and exist upon a continuum, may support better integration of the growing body of literature linking individual sleep dimensions with specific health risks (5–25). In particular, sleep regularity, timing, duration, efficiency, and satisfaction may act independently, additively, or synergistically to influence health risks and outcomes (8).
Research conceptualizing sleep health in a multi-dimensional framework has increased in recent years (19). Matricciani et al. present three methods for examining sleep as a multidimensional construct: analyzing two or more characteristics in a single model (partial least squares regression), the most common approach; creating summary scores; or creating person-centered sleep profiles (using cluster or latent class analysis), which has been utilized to classify sleep quality subtypes among Australian children (26). Recently, Furihata et al. (13) summed self-reported data across five sleep dimensions and found that, after dichotomizing scores into “good” versus “poor” ranges, the sum of “poor scores” was associated with clinical depression. The Osteoporotic Fractures in Men (MrOS) Study (25) similarly assessed seven sleep dimensions, and found that rhythmicity and continuity (minutes awake after sleep onset) were the most robust sleep predictors of mortality (25).
The National Sleep Foundation has previously utilized extensive self-reported data to create a composite Sleep Health index (17). They administered a 28-question telephone interview to a national sample, finding that 14 questions loaded on three factors: sleep duration, sleep quality, and disordered sleep. Sub-indices were combined to create a sleep health index (SHI), and found that stress and overall health were the strongest predictors of SHI, as well as sleep quality in particular.
The current analyses expand upon the limited existing literature on multi-dimensional conceptualizations of sleep health. Most of the extant literature that has examined multiple dimensions of sleep health, has focused on self-reported sleep outcomes, which are subject to bias, and have not focused on populations at high risk for sleep problems.
Similar to Furihata et al. (13), the current study analyzes the relative contributions of individual sleep dimensions, in addition to analyzing an aggregate measure of sleep that sums scores after dichotomizing values on multiple sleep dimensions. Dichotomizing values based on an a priori-defined threshold enables the creation of a single metric that may have clinical relevance, similar to the concept of metabolic syndrome in the cardiovascular literature (27).
While a handful of studies have investigated composite sleep health indices in association with several health outcomes (13,17,25), we extend upon this by examining this question in a sample of low-income African American adults, an under-studied population known to be at substantially higher risk for shorter sleep duration, and poorer sleep quality (5,10,15,16,20), as well as experiencing poorer health (7,12), relative to non-Hispanic Whites. In fact, racial/ethnic differences in sleep duration and quality have recently been proposed as one potential explanation for various physical and mental health disparities (18). Also, it is conceivable that there may be gender differences in associations. One prior study, Osteopathic Fractures in Men (MrOS) (25) focused on men; whereas our sample includes a disproportionate number of women.
The primary aim of these analyses was to investigate the degree to which the composite SH score was associated with physical and mental health outcomes. Because sleep is likely to have different associations with physical versus mental health outcomes, we wanted to include both. Moreover, psychological wellbeing and body mass index are among the most frequently studied risk factors in relation to sleep, which eases comparisons to prior research. We hypothesized that the SH score would be more strongly associated with health than any single sleep dimension. We further hypothesized that having more sleep dimensions in the “healthy” range would be more strongly associated with better health than having fewer sleep dimensions in the “healthy” range. We did not have a priori hypotheses concerning which specific sleep dimensions would be most strongly associated with the health risk factors. Therefore, we consider these analyses exploratory in nature.
Methods
Data for the current analyses come from the Pittsburgh Hill/Homewood Research on Neighborhoods, Sleep, and Health (PHRESH Zzz). This study was designed to investigate changes in the neighborhood environment and health behaviors and risk factors relevant to obesity-related morbidity in two economically disadvantaged, predominantly African American neighborhoods with similar racial-ethnic and socioeconomic characteristics, in Pittsburgh, Pennsylvania. The sample includes randomly selected households from the two neighborhoods, one of which is experiencing multiple economic investments (e.g., housing, greenspace and commercial investment), and the other of which has received substantially less investment. The current study uses data collected between May to November 2013, which we considered baseline data prior to substantial neighborhood investment. Data on sociodemographic characteristics, psychological distress, and physical limitations were collected from an interviewer-administered in-person household survey. Sleep data were collected via actigraphy and sleep diaries.
Participants wore Actigraph GT3X accelerometers 24 hours a day for 7 consecutive days (mean = 5.99 days; SD = 0.66 days) on their wrists, and were required to provide at least four nights of sleep data for inclusion in these analyses. Prior research suggests that a minimum of 4 nights of actigraphy data is recommended to establish reliable sleep parameters via actigraphy (28). Actigraphy data (Actigraph GT3x+) were analyzed using Actilife 6.43 software. Scoring was completed using the Cole-Kripke algorithm (1) and was in line with best practices outlined by the Society of Behavioral Sleep Medicine (6). Sleep windows (i.e., periods between ‘time in bed’ and ‘time out of bed’) were first determined by reported bed/wake times, followed by visual inspection of the actigraphy record. In cases of disagreement between these methods, a manual correction to the reported bed/wake time was implemented based upon the visual pattern. The Actigraph GT3x+ has been validated, with strong significant correlations with polysomnography (PSG) (22), albeit with large standard deviations (22). Actigraph GT3x+ data are equivalent to Actiwatch-64 data (9) for estimating sleep/wake rhythms compared to PSG. Finally, actigraphy data were statistically scanned for extreme cases using ±3 SD both within- and between-persons. These cases, which were few, underwent further visual inspection and were ultimately retained given that estimates were plausible given the actigraphy tracings and we wished not to exclude the most disturbed sleep cases. Actigraphy-assessed sleep dimensions included: regularity, timing, efficiency, and duration. Concurrent with actigraphy, participants filled out sleep diaries every morning upon awakening for the assessment of sleep satisfaction, as well as sleep medication use, which was included as a covariate in all models.
The final analytical sample consisted of 738 participants with complete actigraphy and diary data, out of 828 participants with actigraphy data available. Participants in the analytical sample did not differ significantly from those in the full sample on any of the covariates (e.g., race/ethnicity, gender, age, etc.). Those who were included in the analytical sample had greater actigraphy-measured sleep regularity and later timing, longer sleep duration, and/or lower sleep efficiency (all p-values < 0.05), relative to those who were excluded for missing diary data.
Sleep health (SH) score.
For each of the five sleep dimensions, participants received a score of one to indicate a “healthy” range or zero to indicate an “unhealthy” range. Our approach for defining “healthy” and “unhealthy” ranges on sleep dimensions was to either: 1) use previously published data that have established thresholds for healthy versus unhealthy dimensions (e.g., for sleep duration) or 2) in cases where there are no published criteria to establish thresholds for “healthy” or “unhealthy” ranges we established these thresholds based on the distributions in the current sample (as described below). Therefore, some of these thresholds may be study-specific and will need to be validated in future studies with different populations.
To create the composite SH score, scores from the five sleep measures were summed, resulting in a potential range of 0 to 5, with higher scores indicating greater sleep health. Additionally, five dichotomous variables were created to indicate whether participants scored a 1 (“healthy” sleep) on at least one, two, three, or four or more dimensions (as compared to fewer than one, two, three, four or more dimensions, respectively). These dichotomous variables were then analyzed in a series of five regression models.
Sleep dimensions.
Sleep Duration.
Sleep duration is the total amount of time spent sleeping during the participant’s typical sleep period, assessed by actigraphy. Participants received a score of 1 if their average sleep duration was between 6 and 8 hours, inclusive; and a score of 0 points if their average sleep duration was < 6 hours or > 8 hours. Although much of the literature suggesting an optimal sleep range is between 7 to 9 hours, with sleep durations outside of this range found to be associated with poorer health or increased mortality risk (11,14), these studies are largely based on self-report, which leads to over-estimates compared to actigraphy-assessed sleep. As such, we tested both ranges, found results did not differ substantially, and retained the shorter limits.
Sleep Regularity.
Following prior work, sleep regularity was assessed based on the within-person standard deviation (SD) in actigraphy-assessed sleep duration across all days of the study (21). Participants received a score of 1 if the standard deviation (SD) from their mean sleep duration was less than 60 minutes and a score of 0 if their SD was >= 60 minutes; this value corresponds approximately to the value distinguishing healthy sleepers and individuals with insomnia in a prior study (21).
Sleep Timing.
Sleep midpoint represents the middle of the sleep period between the sleep onset and final awakening, measured by actigraphy. Atypical sleep times, such as those associated with shift work schedules and delayed sleep schedules, are associated with a host of physical and mental health conditions (23). Participants received a score of 0 if their sleep midpoint was after 4:00 AM and 1 if their average sleep midpoint was before 4:00 AM, inclusive. Given that there are currently no clearly defined “healthy” versus “unhealthy” ranges for sleep timing, we based this range on the distribution in our dataset which showed that nearly 64% of participants had midpoints before 4:00 AM. Therefore, the “unhealthy” category included 36% of the population which could be characterized as late midpoints.
Efficiency.
Sleep efficiency represents the total duration of objectively-measured sleep divided by the total time in bed, as reported in sleep diaries and verified by visual inspection of the actigraphy data. Participants received a score of 1 if their level of sleep efficiency was >= 85%, and a score of 0 if their sleep efficiency was <85%. Actigraphy-assessed sleep efficiency less than 85% is associated with poor health outcomes in prior research (29). In insomnia treatment studies, sleep efficiency less than 85% is typically the threshold for defining healthy versus unhealthy sleep and has been used as a quantitative parameter of insomnia (30) (indicating poor sleep quality, or non-response to treatment). Sleep efficiency levels above 85% have also been associated with breast cancer survival in women using wrist-based actigraphy (31).
Sleep Satisfaction.
Diary-assessed sleep satisfaction for each night was rated on a 5-point Likert-scale, ranging from 1 - “very bad” to 5 - “very good.” Participants received a score of 1 if their average sleep satisfaction was >=4 (good to very good sleep) and a score of 0 if their average sleep satisfaction was < =3 (i.e., “neutral” to “very poor”).
Psychological distress.
The Kessler 6 (K6), a well-validated self-report instrument was used to assess general psychological distress (32). The questionnaire asked respondents, “During the last 30 days, about how often did …” “…you feel hopeless?”; “…you feel restless or fidgety?”; “…you feel that everything was an effort?” “…you feel nervous?”; “… you feel worthless?” Higher scores are indicative of greater distress, with scores >=13 being indicative of clinically significant distress (33). Symptoms were first rated on a scale from 0 (none of the time) to 4 (all of the time), and ratings were summed to create a composite score with possible scores ranging from 0 to 24. Internal consistency is high (Cronbach’s 〈= 0.85).
Functional limitations.
Participants reported on the extent to which they experience physical limitations in ten different physical activities, using the Physical Limitations Scale from the Medical Outcomes Study Short-Form Health Survey (SF-36) (34). Specifically, participants reported on whether their health limited them “a lot,” “a little,” or not at all.” The subscale scores range from 0 to 100, with higher scores indicating better physical health. The reliability and validity of the SF-36 and the physical limitations subscale have been extensively documented in past research (35–38).
Body mass index.
BMI (kg/m2) was calculated from measured height and weight. Interviewers measured participants’ height to the nearest eighth-inch using a carpenter’s square (triangle) and an eight-foot folding wooden ruler marked in inches. Weight was measured to the nearest tenth-pound using a Seca Robusta 813 digital scale.
Covariates.
Sociodemographic characteristics and study site/neighborhood (Hill District or Homewood neighborhoods) were included as covariates to examine the independent association between sleep dimensions or the SH score and health outcomes. Individual-level socioeconomic status (SES) was captured by participant-reported educational attainment and annual per capita household income. Race/ethnicity was derived from two self-reported items, one on race and the other on ethnicity (i.e., Hispanic, non-Hispanic). Additional covariates were: age, gender, marital/cohabitation status, and presence of children in the home. As part of the daily diary, participants were asked, “Did you take any sleep medication to help you fall asleep tonight? (yes or no).” Based on this item, a binary measure of use of any sleep medication was also included as a covariate.
Analytic Strategy.
Multivariable linear regression analyses were conducted to investigate whether individual sleep dimensions, or the composite SH score, were related to psychological distress, physical functioning, and BMI (in separate models), after adjusting for covariates. Next, we estimated three models (one for each health outcome) in which we entered all five continuous sleep dimensions into the models in order to assess the relative contributions of each individual sleep dimension, while adjusting for the other four. Correlations between individual sleep dimensions ranged from a low of r = −0.0002 for duration and timing (to a high of r = 0.52 for efficiency and duration, suggesting that multi-collinearity is not an issue in these models.
Additionally, in sensitivity analyses, we also analyzed the sleep score excluding self-reported sleep satisfaction to avoid the possibility that psychological distress, physical functioning, and sleep satisfaction, which all come from survey reports, may share a common source of bias driving all the results. Due to the large number of significance tests conducted in our analyses and subsequent risk of inflated type I error, we adjusted significance levels for multiple testing using the Benjamini-Hochberg method to provide a more rigorous approach than use of the conventional p < 0.05 standard (39). We only report results that are significant after multiple testing adjustment.
Results
Descriptive statistics for the analytic sample are presented in Table 1. Participants were on average 55 years old, with a mean per capita annual household income of $13,000. Ninety-eight percent of the sample identified as African American, and males constituted 22% of the sample. Nearly 17% of participants reported taking sleep medications. Participants had an average BMI of 31.2 (SD= 7.4), and physical functioning score of 65.9 (SD = 28.8) on a scale of 0 to 100. Their average level of psychological distress was 4.3 (SD = 4.6) on a scale of 0 to 24, and 7% had a score >= 13, which is considered clinically significant and warranting intervention for mental illness. Table 1 also shows the percentage of people with scores in the “healthy” range for each sleep dimension.
Table 1:
Summary of Sample Characteristics (N = 738)
| Mean (SD) or Percentage | ||
|---|---|---|
| Sociodemographics | ||
| Age (years) | 55.4 (16.2) | |
| Male | 22% | |
| African American | 98% | |
| Household income per capita (in $1000s) | 13.0 (12.6) | |
| Any children in home | 28% | |
| Neighborhood Hill District | 65% | |
| Taking any sleep medications | 17% | |
| Health indicators | ||
| Body mass index | 31.2 (7.4) | |
| Psychological distress | 4.3 (4.6) | |
| Physical functioning | 65.9 (28.8) | |
| Sleep Measures | % “Good” range | |
| Regularity | 62% | |
| Satisfaction | 44% | |
| Timing (sleep period midpoint) | 64% | |
| Sleep Efficiency (in %) | 37% | |
| Duration (in hours) | 41% | |
Notes: Regularity= standard deviation of sleep duration in hours; Timing = sleep period midpoint; Sleep Efficiency = hours asleep/hours in bed; Duration = Total sleep time in hours
Satisfaction is measured on a response scale 1–5 in daily diaries. All the other sleep variables were assessed via actigraphy data.
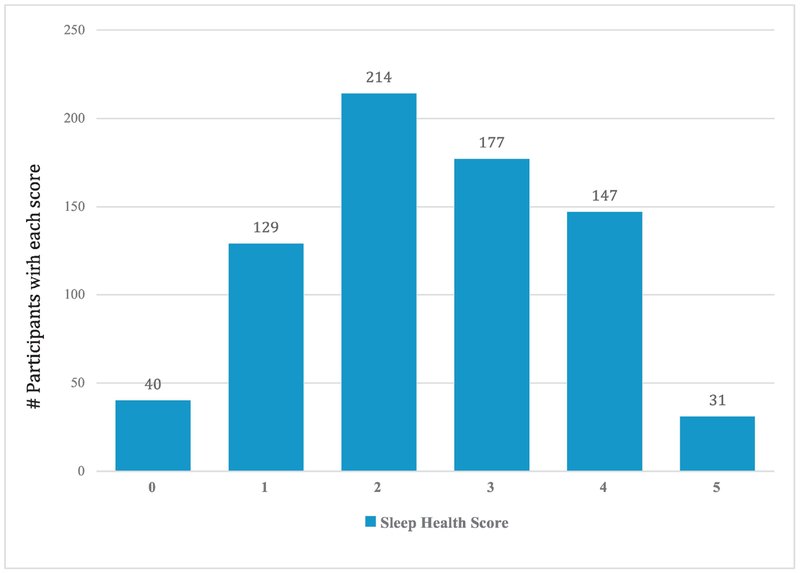
Table 2 shows distributions for the continuous sleep variables. Mean sleep length was 5.99 hours (SD = 1.41), and half of study participants had sleep times with midpoints of 3:39 AM or earlier. Mean sleep efficiency was 80.5% (SD = 9.8%). Half the sample had within-person day-to-day variation in sleep duration (SD) of 51.0 minutes or less, with an average of 66 minutes (SD = 29). Finally, the most frequently reported self-reported sleep satisfaction score was 3, (mean = 3.74; SD = 0.78) on a scale of 1 to 5. Figure 1 shows the distribution of SH scores.
Table 2:
Summary of Sleep Dimensions in Sample (N = 738)
| Minimum | 25th percentile |
50th percentile |
75th Percentile |
Maximum | |
|---|---|---|---|---|---|
| Individual dimensions | |||||
| Regularity (SD in hours) | 0.0 | 0.64 | 1.08 | 1.36 | 5.95 |
| Satisfactiona | 1.0 | 3.17 | 3.74 | 4.29 | 5.0 |
| Timing (sleep period midpoint) | 11:22pm | 2:43 am | 3:39 am | 4:28 am | 10:36 am |
| Sleep Efficiency (in %) | 35.76 | 74.97 | 80.5 | 87.01 | 99.95 |
| Duration (in hours) | 2.67 | 6.42 | 7.45 | 8.42 | 12.34 |
| Composite score | |||||
| Sleep health scoreb | 0.0 | 1 | 2 | 3 | 5.0 |
Notes: Regularity= SD sleep duration in hours; Timing = sleep period midpoint; Sleep Efficiency = hours asleep/hours in bed; Duration = Total sleep time in hours
Satisfaction is measured on a self-reported response scale 1–5; All other sleep variables are based on actigraphy data.
Sleep health can range from 0 to 5.
Figure 1.
Percentage of participants with sleep in the “better” range: A multi-dimensional sleep health score (Satisfaction, Duration, Timing, Efficiency, Regularity)
Regression models.
Associations of sleep health score with health outcomes.
BMI.
Neither the continuous SH score, nor the individual SH variables that divide the continuous SH score into five dichotomous variables (≥1 vs. 0, etc.), were significantly associated with BMI after adjustment for multiple comparisons (Table 3). Hence, the tables focus on associations of sleep with psychological distress and physical functioning.
Table 3.
Associations of Five Sleep Dimensions (Satisfaction, Duration, Timing, Efficiency, Regularity) with Psychological Distress and Physical Functioning
| Sleep Dimensions | B (SE) |
|---|---|
| Psychological Distress | |
| 1. SH score (count) | −0.55(0.13)* |
| 2. SH Score ≥1 | −0.59(0.71) |
| 3. SH Score ≥2 | −1.09(0.39)* |
| 4. SH Score ≥3 | −1.36(0.33)* |
| 5. SH Score ≥4 | −1.25(0.38)* |
| Physical Functioning | |
| 1. SH score (count) | 2.23(0.78)* |
| 2. SH Score ≥1 | 3.10(4.22) |
| 3. SH Score ≥2 | 3.53(2.30) |
| 4. SH Score ≥3 | 4.66(1.95) |
| 5. SH Score ≥4 | 6.91(2.26)* |
| BMI | |
| 1. SH score (count) | −0.07(0.22) |
| 2. SH Score ≥1 | −0.60(1.19) |
| 3. SH Score ≥2 | −0.38(0.65) |
| 4. SH Score ≥3 | −0.05(0.55) |
| 5. SH Score ≥4 | −0.54(0.64) |
Notes:
a Results are significant after Benjamini-Hochberg multiple testing adjustment with the false discovery rate set to 5%.
Each row represents a different model. Models 2 through 5 compare having a sleep health (SH) score of at least X number (stated in model) versus a lower SH score. Sleep health scores range from 0 to 5.
All models include as covariates: age, race, gender, marital status, household income, educational attainment, neighborhood, presence of children in the home, and sleep medication use.
Psychological distress. SH Score.
Associations of the continuous SH score with psychological distress are shown in Table 3. The continuous SH score was significantly associated with psychological distress such that each 1-unit increase on the sleep health score was associated with a −0.55 (SE = 0.13, p < 0.05; effect size = −0.151) lower psychological distress score, after adjustment for covariates and multiple testing.
Psychological distress. Dichotomous Sleep Health Scores.
Next, dichotomous versions of SH score levels (e.g., ≥1 versus 0; ≥2 versus 0 or 1, etc.), were also examined in separate models. We divided the composite SH score into 5 categories (0 to 4+) to determine whether there was a specific cutoff point (corresponding to the number of sleep dimensions in the “healthy” range) at which associations were most evident. Individuals with SH scores ≥2, ≥3, or ≥4 had lower levels of psychological distress than persons with a lower SH scores, with associations corresponding to reductions of 1.09, 1.36, and 1.25 points, respectively. Effect sizes were −0.24, −0.30, and −0.27 for persons with SH scores ≥2, ≥3, or ≥4, respectively.
Physical functioning.
The continuous SH score was also significantly associated with physical functioning (table 3). Each 1-unit increase on the sleep health score was associated with a 2.23 (SE = 0.78, p < 0.05; effect size = 0.098) higher physical functioning score. Moreover, having a sleep health score of at least four (versus lower scores), was significantly associated with a 6.91-unit higher physical functioning score (SE = 2.26, p < 0.05; effect size = 2.26).
Individual and joint associations of continuous sleep dimensions with health outcomes.
BMI.
None of the individual sleep dimensions were significantly associated with BMI in any of the models (Table 3).
Psychological Distress:
We looked at associations of continuous sleep dimensions with psychological distress individually, estimated as five separate models including a single sleep health dimension at a time, as well as in a joint model with all 5 sleep dimensions entered at once. Entered individually, only sleep satisfaction was significantly associated with psychological distress (® = 1.58, SE = 0.20). When all five sleep dimensions were entered simultaneously into a single model, sleep satisfaction (® = 1.55, SE = 0.21) continued to be associated with psychological distress after adjustment for the other sleep dimensions (Table 4).
Table 4.
Adjusted Linear Regressions of Continuous Sleep Dimensions with Psychological Distress and Physical Functioning
| Psychological Distress | Physical Functioning | BMI | |
|---|---|---|---|
| Sleep dimensions | B (SE) | B (SE) | B (SE) |
| Sleep Satisfaction | −1.55(0.21)* | 4.46(1.24)* | −0.72(0.55) |
| Duration | −0.02(0.13) | −2.44(0.80)* | 0.38(0.56) |
| Regularity | 0.10(0.29) | −2.15(1.77) | 1.46(0.56) |
| Efficiency | −0.02(0.02) | 0.22(0.12) | −0.83(0.58) |
| Timing | −0.13(0.11) | −1.81(0.69)* | −0.66(0.57) |
Notes:
Results are significant after Benjamini-Hochberg multiple testing adjustment with the false discovery rate set to 5%.
The models enter all five sleep variables simultaneously, using the continuous variables for each sleep dimension. All models include as covariates: age, race, gender, marital status, household income, educational attainment, neighborhood, presence of children in the home, and sleep medication use. Self-reported response scale 1–5. All other variables are based on actigraphy data.
Physical functioning.
Table 4 shows associations of each sleep dimension with physical functioning, both independently and after adjustment for the other four sleep dimensions. When each sleep dimension was analyzed individually, sleep satisfaction (® = 4.59, SE = 1.23) and timing (® = −2.27, SE = 0.69) were both significantly associated with physical functioning, such that better sleep satisfaction and earlier timing were associated with better physical functioning. When all five sleep dimensions were analyzed in a joint model, sleep satisfaction (® = 4.46, SE = 1.24), duration (® = −2.44, SE = 0.80), and timing (® = −1.81, SE = 0.69) were all significantly associated with physical functioning, indicating that higher sleep satisfaction, earlier sleep timing, and shorter duration were all associated with better physical functioning.
In sensitivity analyses, we also analyzed the sleep score excluding self-reported sleep satisfaction. We found results were similar, although existing significant associations were slightly stronger with the shorter range in some models.
Discussion
Within our study population of low-income African Americans living in two urban neighborhoods, the SH score representing a combination of sleep regularity, timing, duration, efficiency, and satisfaction was significantly associated with psychological distress, such that higher levels of sleep health were associated with lower levels of psychological distress. Additionally, three sleep dimensions were associated with physical functioning when analyzed simultaneously in a single model. Consistent with our hypotheses, the continuous SH score was a stronger predictor of psychological distress than any individual sleep dimension, with the exception of self-reported sleep satisfaction. Moreover, having more sleep dimensions within the “healthy” range was also significantly associated with lower levels of psychological distress and higher levels of physical functioning than having fewer sleep dimensions in this range. Finally, sleep satisfaction was also independently associated with lower psychological distress.
Contrary to our hypotheses, neither the SH score nor any of the individual sleep dimensions were significantly associated with BMI. Other studies in adults have found significant associations (40). However, the lack of association with BMI replicates prior work in the National Longitudinal Study of Adolescent Health finding that sleep duration and BMI were not associated in African American males and positively associated in African American females (41). Moreover, associations of sleep with BMI tend to be stronger in children and younger adults than in older adults (42). The current study includes a relatively older adult population, as compared to the general population which could contribute to weaker than expected associations.
Although only one of the dimensions (satisfaction) was significantly associated with psychological distress, the composite sleep health score was associated with psychological distress in a near-linear fashion. This suggests that there may be a cumulative effect of having poorer sleep health across a range of dimensions. Moreover, both the analyses of associations of sleep health with psychological distress (where the composite score and satisfaction alone was associated) and physical functioning (where both the composite score, as well as three sleep individual dimensions were associated) speak to the importance of measuring multiple sleep dimensions, in addition to creating a composite index that accounts for all simultaneously.
While several prior studies have examined associations of sleep disturbances with physical limitations (43,44), previous studies have not focused on high-risk samples, nor have they tested for effect modification by race/ethnicity. Although the composite SH score was not associated with physical functioning, higher sleep satisfaction and earlier sleep timing were associated with higher physical functioning. Additionally, poorer physical functioning was also associated with longer sleep duration, which may reflect greater physical health morbidity among long sleepers, as well as poorer physical functioning. The fact that three of the sleep dimensions were significantly associated with physical functioning when entered in the same model speaks to the unique contributions of each dimension to health, and as such, the importance of measuring each.
Sleep duration was not a significant predictor of psychological distress or BMI in the current study, despite frequently being the sole sleep measure assessed in many studies. In addition, while subjectively-assessed sleep satisfaction was strongly associated with psychological distress, none of the individual objectively-assessed sleep dimensions were associated with psychological distress or BMI.
Despite these strengths, the current study is limited by its use of cross-sectional data, as associations could be bi-directional in nature and/or both sleep and health could be influenced by additional, unobserved factors. In addition, the racial homogeneity, the over-representation of women, and the low variability in SES of the study sample could limit the generalizability of current findings to other populations, including non-Hispanic Whites, younger people, males, and persons of higher SES. Specifically, findings may be stronger in a lower risk sample. In addition, while our composite index represents five key dimensions of sleep health, we did not have a measure of daytime functioning, such as alertness, which is also considered to be an important dimension of sleep health (8). Furthermore, we utilized a specific approach to operationalize the sleep health index, based on a count of binary scores, as this approach may have clinical utility. However, other approaches may also be useful and warrant further study. Furthermore, although results may differ with different cutoffs, we did test different cutoffs for duration and timing and did not find substantial differences. However, we selected these cutoffs based on values previously found to be associated with health and believe that this makes for the most straightforward analysis and simplifies potential replication.
Additionally, equal weighting of all sleep measures and use of linear scoring are a limitation of this study (as well as any others that attempt to develop composite scores the combine data from multiple indices). However, this is a relatively homogenous, although large, sample. We aimed to provide a relatively straightforward analysis – to be replicated and perhaps eventually modified using more complex measures in a more representative study sample.
These limitations notwithstanding, the current study provides a number of contributions to our understanding of sleep-health associations. First, we included both subjective and objective measures of multiple sleep dimensions and analyze these in relation to multiple physical and mental health risk factors. Furthermore, we explored these associations in a large sample of low income, African American adults who, despite being at risk for shorter sleep duration, poorer sleep satisfaction, and poorer cardio-metabolic health, are underrepresented in studies of sleep-health associations. We also include a predominantly female sample, in contrast to the MrOS sample (25), who similarly created a sleep health index to predict clinical depression risk. Our inclusion of objectively-assessed data on a disparate range of key sleep dimensions is novel and advances understanding of the extent to which these specific sleep dimensions may relate to health.
In summary, the current findings add to a growing body of literature which highlights the importance of considering the independent and additive effects of multiple dimensions of sleep on important health indicators in a high-risk sample. The current findings offer a specific approach to operationalizing sleep health, in a clinically meaningful way. Future research should investigate different operationalizations of sleep health, and whether associations with the current SH score and health outcomes are generalizable to other populations.
Supplementary Material
Acknowledgments
Support for the study which serves as the source of these data was provided by the National Heart, Lung & Blood Institute (R01HL122460) and the National Cancer Institute (R01CA164137).
Disclosure statement
• Dr. Buysse reports receiving consultation fees from BeHealth Solutions, Emmi Solutions, and CME Institute; and grants from NIH, outside the submitted work. In addition, Dr. Buysse receives licensing fees (royalties) for the Pittsburgh Sleep Quality Index (PSQI), the Daytime Insomnia Symptoms Scale (DISS), and the Consensus Sleep Diary (CSD), which are copyrighted and/or licensed by the University of Pittsburgh.
• Dr. Hale serves on the Board of Directors of the National Sleep Foundation, and receives an honorarium for her role as Editor-in-Chief of this journal, Sleep Health.
• No other authors report any conflicts of interest. Our funding disclosures are included in the acknowledgments.
Abbreviations list:
- BMI
body mass index
- SH
Sleep health
- PSG
polysomnography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
• All authors have seen and approved this manuscript.
• The manuscript does not report on a clinical trial
References
- 1.Irwin MR. Why sleep is important for health: A psychoneuroimmunology perspective. Psychology. 2015;66(1):143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity. 2008;16(3):643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snell EK, Adam EK, Duncan GJ. Sleep and the body mass index and overweight status of children and adolescents. Child Dev. 2007;78(1):309–323. [DOI] [PubMed] [Google Scholar]
- 4.Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Arch Intern Med (Chic). 2006;166(16):1689–1692. [DOI] [PubMed] [Google Scholar]
- 5.Adam EK, Snell EK, Pendry P. Sleep timing and quantity in ecological and family context: A nationally representative time-diary study. J Fam Psychol. 2007;21(1):4. [DOI] [PubMed] [Google Scholar]
- 6.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: Clinical and research applications. Behav Sleep Med. 2015;13(sup1):S4–S38. [DOI] [PubMed] [Google Scholar]
- 7.Beckles GL, Zhu J, Moonesinghe R. Diabetes-United States, 2004 and 2008. MMWR Surveill Summ. 2011;60(Suppl):90–93. [PubMed] [Google Scholar]
- 8.Buysse DJ. Sleep health: Can we define it? Does it matter. Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cellini N, McDevitt EA, Mednick SC, Buman MP. Free-living cross-comparison of two wearable monitors for sleep and physical activity in healthy young adults. Physiol Behav. 2016;157:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among US adults. Obesity. 2014;22(2):598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman DS. Obesity—United States, 1988–2008. MMWR Surveill Summ. 2011;60(01):73–77. [PubMed] [Google Scholar]
- 13.Furihata R, Hall MH, Stone KL, et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep. 2017;40(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: The Sleep Heart Health Study. Sleep. 2006;29(8):1009. [DOI] [PubMed] [Google Scholar]
- 15.Grandner MA, Petrov M, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9(9):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hale L, Do P. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutson KL, Phelan J, Paskow MJ, et al. The National Sleep Foundation’s sleep health index. Sleep Health. 2017;3(4):234–240. [DOI] [PubMed] [Google Scholar]
- 18.Laposky AD, Van Cauter E, Diez-Roux AV. Reducing health disparities: The role of sleep deficiency and sleep disorders. Sleep Med. 2016;18:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matricciani L, Bin YS, Lallukka T, et al. Rethinking the sleep-health link. Sleep Health. 2018;4(4):339–348. [DOI] [PubMed] [Google Scholar]
- 20.Matthews KA, Hall M, Dahl RE. Sleep in healthy black and white adolescents. Pediatr. 2014;133(5):e1189–e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okun ML, Reynolds III CF, Buysse DJ, et al. Sleep variability, health-related practices and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73(2):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR. Assessing sleep using hip and wrist actigraphy. Sleep Biol Rhythms. 2015;13(2):172–180. [Google Scholar]
- 23.Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. Br Med J. 2012;345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walch OJ, Cochran A, Forger DB. A global quantification of “normal” sleep schedules using smartphone data. Sci Adv. 2016;2(5):e1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace ML, Stone K, Smagula SF, et al. Which Sleep Health Characteristics Predict All-Cause Mortality in Older Men? An Application of Flexible Multivariable Approaches. Sleep. 2017;41(1):zsx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magee CA, Robinson L, Keane C. Sleep quality subtypes predict health-related quality of life in children. Sleep Med. 2017;35:67–73. [DOI] [PubMed] [Google Scholar]
- 27.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26(3):337–341. [DOI] [PubMed] [Google Scholar]
- 29.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 31.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41(4):427–445. [DOI] [PubMed] [Google Scholar]
- 32.Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32(06):959–976. [DOI] [PubMed] [Google Scholar]
- 33.Forman-Hoffman VL, Muhuri PK, Novak SP, Pemberton MR, Ault KL, Mannix D. Psychological distress and mortality among adults in the US household population. CBHSQ Data Review. 2014. [Google Scholar]
- 34.McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993:247–263. [DOI] [PubMed] [Google Scholar]
- 35.Brazier JE, Harper R, Jones N, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Bmj. 1992;305(6846):160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchwald D, Pearlman T, Umali J, Schmaling K, Katon W. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am J Med. 1996;101(4):364–370. [DOI] [PubMed] [Google Scholar]
- 37.Stansfeld S, Roberts R, Foot S. Assessing the validity of the SF-36 General Health Survey. Qual Life Res. 1997;6(3). [DOI] [PubMed] [Google Scholar]
- 38.Ware JE Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995:AS264–AS279. [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995:289–300. [Google Scholar]
- 40.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev. 2012;16(3):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reither EN, Krueger PM, Hale L, Reiter EM, Peppard PE. Ethnic variation in the association between sleep and body mass among US adolescents. Int J Obes (Lond). 2014;38(7):944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandner MA, Schopfer EA, Sands-Lincoln M, Jackson N, Malhotra A. Relationship between sleep duration and body mass index depends on age. Obesity. 2015;23(12):2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30(10):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21(1):27–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.