Figure 7. Blocking the activity of SCD1 with small molecule inhibitors increases lipid peroxidation, ferroptotic cell death, and the anti-tumor effect of a ferroptosis inducer in vivo.
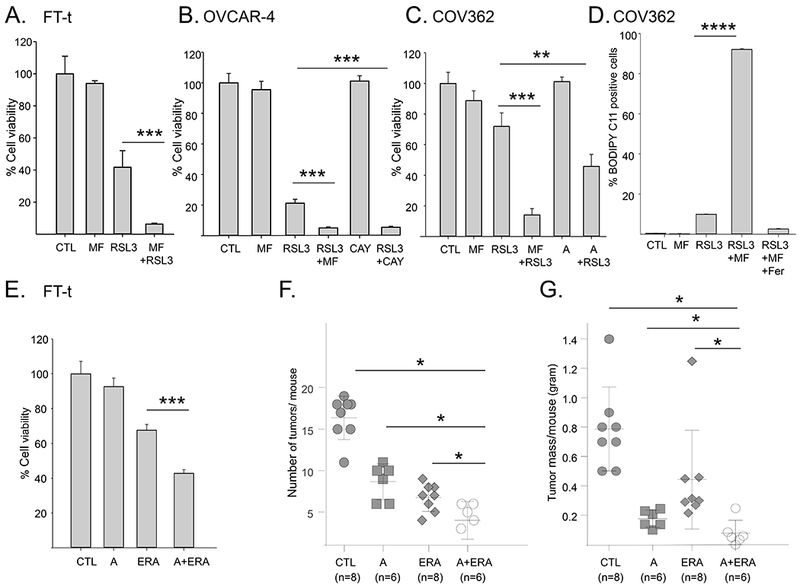
A-C. Viability of FT-t, COV362, or OVCAR-4 cells pretreated with 1μM MF-438 (MF), 1 μM CAY10566 (CAY), or 5 μM A939572 (A) for 24 hours followed by the addition of RSL3 for an additional 24 hours. For each cell type, dose of RSL3 was adjusted to attain modest cytotoxicity in the absence of SCD1 inhibitors (FT-t: 2 μM RSL3;OVCAR-4:1μM RSL3; COV362: 5 μM RSL3). Control cells were treated with SCD1 inhibitor for 48 hours or RSL3 for 24 hours. Data shown is representative of at least three independent experiments each with 8 to 12 replicas D. C11-BODIPY staining of COV362 cells treated with 1 μM MF-438 for 24 hrs followed by 1 μM RSL3 for 4 hrs in the presence or absence of Fer1. E. Viability of FT-t cells treated with A939572 (A) alone, 1μM erastin (ERA) alone, or A939572 followed by erastin. Graphs are representative of at least three independent experiments each with 3 to 8 replicas. F,G. Mice were injected intraperitoneally with FT-t cells and treated for 18 days with either vehicle control, A939572 (A), erastin (E) or the combination of A939572 and erastin. (F) depicts the number of tumors/mouse and (G) depicts total tumor mass/mouse. *p<0.04; **p<0.00027; ***p<2 E-05; ****p<7.2 E-10.
