Figure 8.
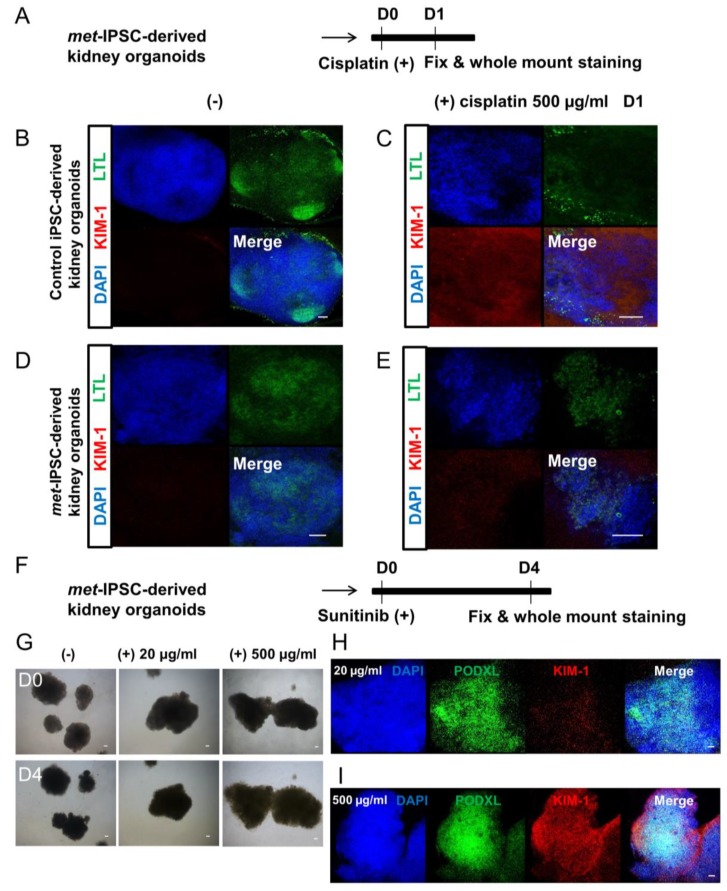
Figure 8. Drug toxicity experiments. (A) Schematic representation of cisplatin toxicity experiments in met-IPSC kidney embryoid bodies. (B–C) Representative whole-mount immunostaining for LTL, KIM-1 and DAPI in control iPSC-derived kidney embryoid bodies treated with cisplatin (500 µg/mL) (right panels). (D–E) Representative whole-mount immunostaining for LTL, KIM-1 and DAPI in met-IPSC-derived kidney embryoid bodies treated with cisplatin (500 µg/mL) (right panels). (F) Schematic of drug Sunitinib toxicity test process of met-IPSC-derived kidney embryoid bodies. (G) Photograph of met-IPSC-derived kidney embryoid bodies in 96 well plate. (H) Representative whole-mount immunostaining for PODXL, KIM-1 and DAPI in met-IPSC-derived kidney embryoid bodies treated with Sunitinib (20 µg/mL). (I) Representative whole-mount immunostaining for PODXL, KIM-1 and DAPI in met-IPSC-derived kidney embryoid bodies treated with Sunitinib (500 µg/mL), Scale bar: 100 μm.