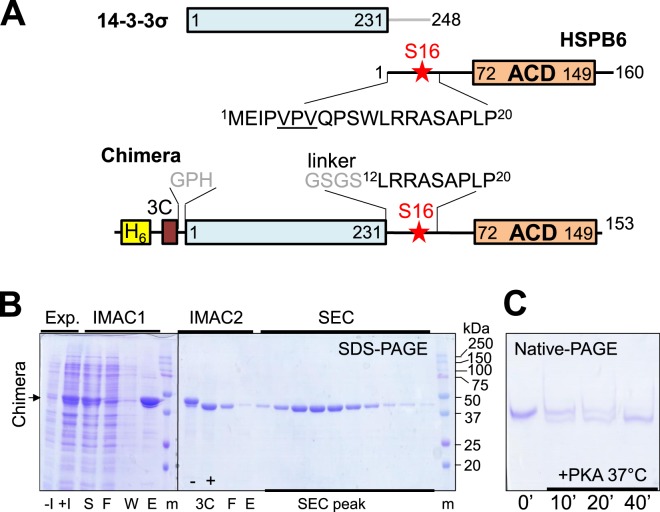
Figure 1.
Design and purification of the 14-3-3-pB6 chimera. (A) Schematics showing the primary structure of both individual proteins and the chimera. The N-terminal hexahistidine-tag cleavable by 3C protease is shown. (B) Expression (Exp.) and chromatographic (IMAC1, IMAC2, SEC) purification of the unphosphorylated chimera as analyzed by SDS-PAGE. Qualitatively similar results were obtained after co-expression with PKA, but with a lower yield (not shown). Non-induced (−I), induced (+I), soluble (S), flowthrough (F), wash (W), eluted (E) fractions, and fractions collected during SEC (SEC peak) are indicated below the gel. Protein markers (m) with the corresponding masses (in kDa) are also indicated. Position of the chimera band is shown by an arrow to the left. Note the shift after the 3C proteolysis (3C: “−”, “+”). (C) Kinetics of in vitro phosphorylation of the purified chimera by PKA analyzed by native-PAGE. Note the downward shift corresponding to phosphate group incorporation.