Fig. 1.
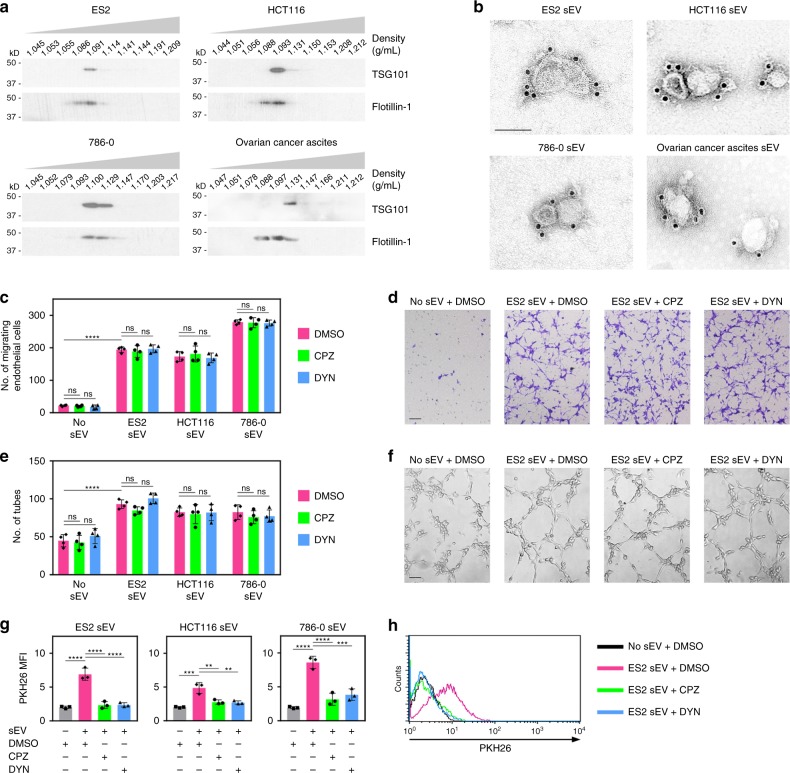
Cancer cell-derived sEVs can stimulate endothelial cell migration and tube formation independently of uptake. a Immunoblot of TSG101 and flotillin-1 in fractions of the indicated buoyant densities that were isolated from media conditioned by ovarian (ES2), colorectal (HCT116), and renal (786-0) cancer cell lines, and from ovarian cancer patient ascites. b Immunogold labeling of CD63 on vesicles in sEV fractions (i.e., density of 1.09–1.13 g/mL). Scale bar = 100 nm. c–f Human umbilical vein endothelial cells (HUVEC) were pretreated with endocytosis inhibitors (chlorpromazine, CPZ; dynasore, DYN) or with dimethyl sulfoxide (DMSO) solvent, and then stimulated with sEVs of ES2, HCT116, and 786-0 cells. Shown are numbers (c) and representative images (d) of migrating HUVEC at 5 h after stimulation, and numbers (e) and representative images (f) of tubes formed at 4 h after stimulation. Mean ± SD of n = 4 independent experiments are shown. Scale bar = 100 μm. g, h HUVEC were treated as in c and evaluated for uptake of PKH26 dye-labeled sEVs by flow cytometry at 5 h thereafter. Shown are mean fluorescence intensity (MFI) values of PKH26 fluorescence detected in HUVEC in n = 3 independent experiments (g) and representative histogram plots (h). Gating strategy and contour plots are shown in Supplementary Fig. 3b and 4b, respectively. **P < 0.01, ***P < 0.001, ****P < 0.0001, by ANOVA with Bonferroni’s corrections; one-way in g, two-way in c and e. ns: not significant. Source data used for graphs in c, e, and g can be found in Supplementary Data 1