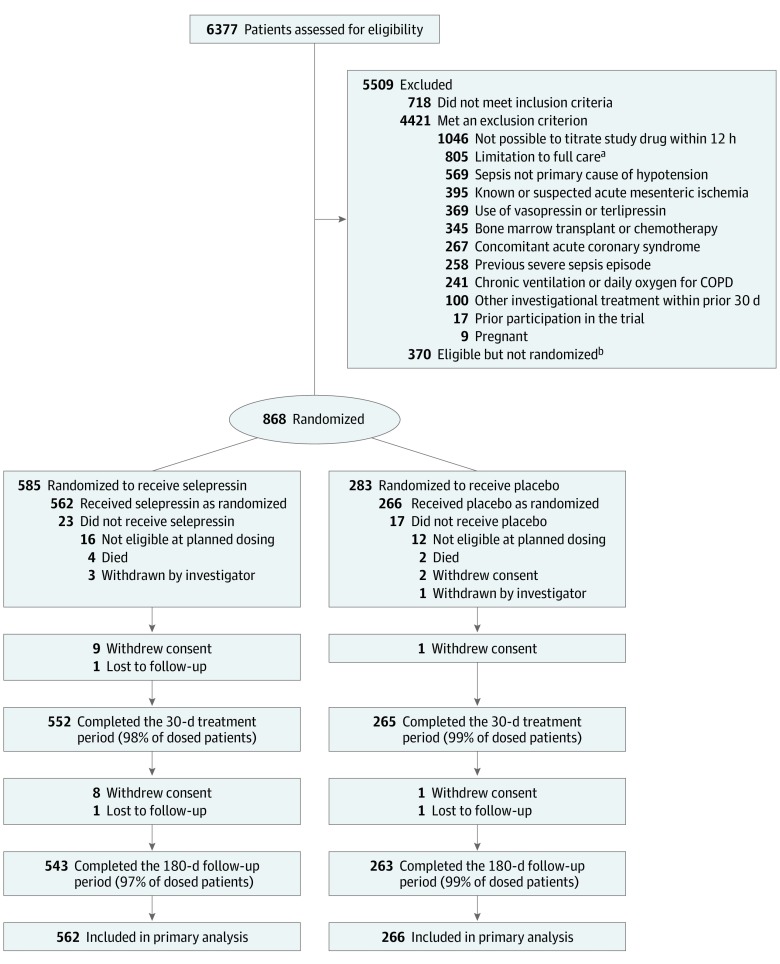
Figure 1. Screening, Randomization, and Follow-up of a Study of Selepressin for Septic Shock.
Patient disposition from screening through day 180 is shown. Efficacy and adverse event analyses are based on all patients who underwent randomization and received selepressin or placebo. Just before dosing, all randomized patients had to fulfill 3 predefined criteria to be eligible for receiving study drug: (1) had received a minimum of 30 mL/kg fluid in total from onset of hypotension (or less if fluid replete/overload); (2) still receiving a continuous infusion of 5 µg/min or more of norepinephrine and having done so for at least 1 hour; and (3) less than 12 hours had passed from start of vasopressor treatment for sepsis. COPD indicates chronic obstructive pulmonary disease.
aLimitations to full care included any decision to limit care, including lack of commitment to cardiopulmonary resuscitation, blood products, kidney replacement therapy, surgery to ensure source control, or mechanical ventilation if indicated.
bReasons for failure to enroll despite meeting eligibility criteria included lack of availability of study personnel or study drug and difficulty communicating with the coordinating center.