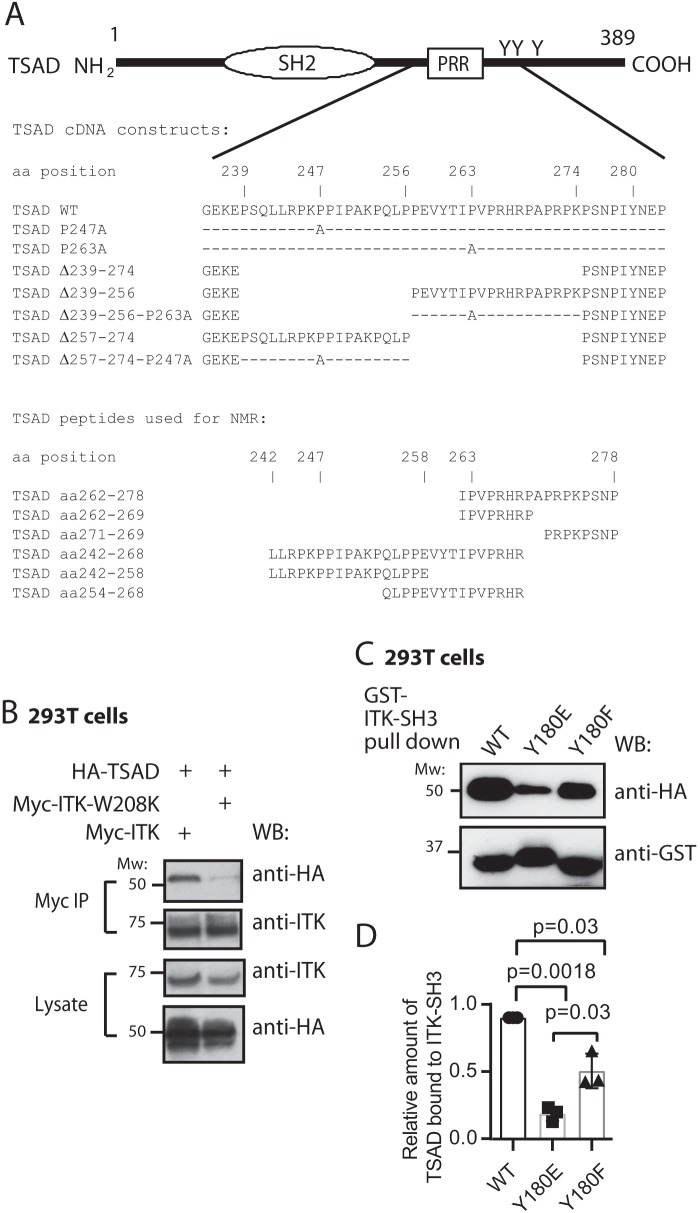
Figure 1.
Nonphosphorylated ITK SH3 domain is required for ITK binding to TSAD. A, schematic drawing of TSAD including the SH2 domain, the PRR and the three C-terminal tyrosines. The core sequences encoded by the TSAD cDNA constructs and the TSAD peptides used for transfection of cells and for NMR experiments, respectively, in this paper are also indicated. B, co-immunoprecipitation experiment showing the dependence of ITK-TSAD interaction on the SH3 domain of ITK. 293T cells were transfected with the indicated cDNA plasmids. Myc-tagged ITK proteins were immunoprecipitated from the cell lysates, followed by immunoblotting with the indicated antibodies. The result is one representative of two experiments. C and D, ITK SH3 domains mutated for Tyr180 display reduced interaction with TSAD. C, pulldown experiment using ITK SH3 domains with the indicated mutations was performed using lysates of 293T cells transiently transfected with HA-tagged WT and mutated TSAD cDNA. Pulled down proteins were immunoblotted with the indicated antibodies. D, the graph represents relative amount of TSAD interacting with ITK SH3 in the experiment shown in C. Signals were quantified by ImageJ analysis (n = 3, mean ± S.D. (error bars)).