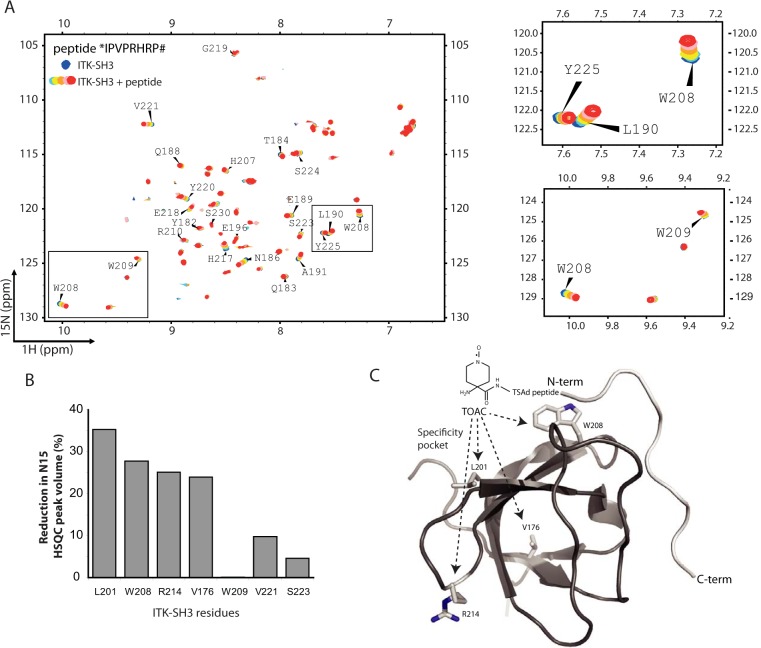
Figure 4.
The human ITK SH3 domain binds to TSAD aa 262–269 in a class I orientation. A, 1H-15N HSQC of human 20 μm ITK SH3 domain without (blue) and with increasing amounts (200 μm to 1 mm, light blue to green-red) of TSAD aa 262–269 (*IPVPRHRP#) added. Chemical shift changes are observed indicating binding of the peptide to the ITK SH3 domain. B, TOAC experiment. HSQC experiments using TSAD aa 262–269 with an N-terminal TOAC aa were performed with and without a reducing agent (ascorbate) added to the solution to remove the effect of the TOAC aa. TOAC aa causes broadening of peaks in the NMR spectrum representing neighboring aa. The graph shows the percentage reduction in peak volume of the four most affected aa (Leu201, Trp208, Arg214, and Val176) in addition to three aa (Trp209, Val221 and Ser223) that the titration experiments had identified to be affected by peptide binding. C, three-dimensional structure of human ITK SH3 domain with the TOAC-labeled TSAD peptide aa 262–269 (light gray) docked onto the SH3 domain using constraints given by the aa most affected by the peptide titration. The N and C termini of the TSAD peptide are labeled N-term and C-term, respectively. The location of Leu201, Trp208, Arg214, and Val176 (resonances most affected by TOAC) are shown on the SH3 structure.