Figure 5.
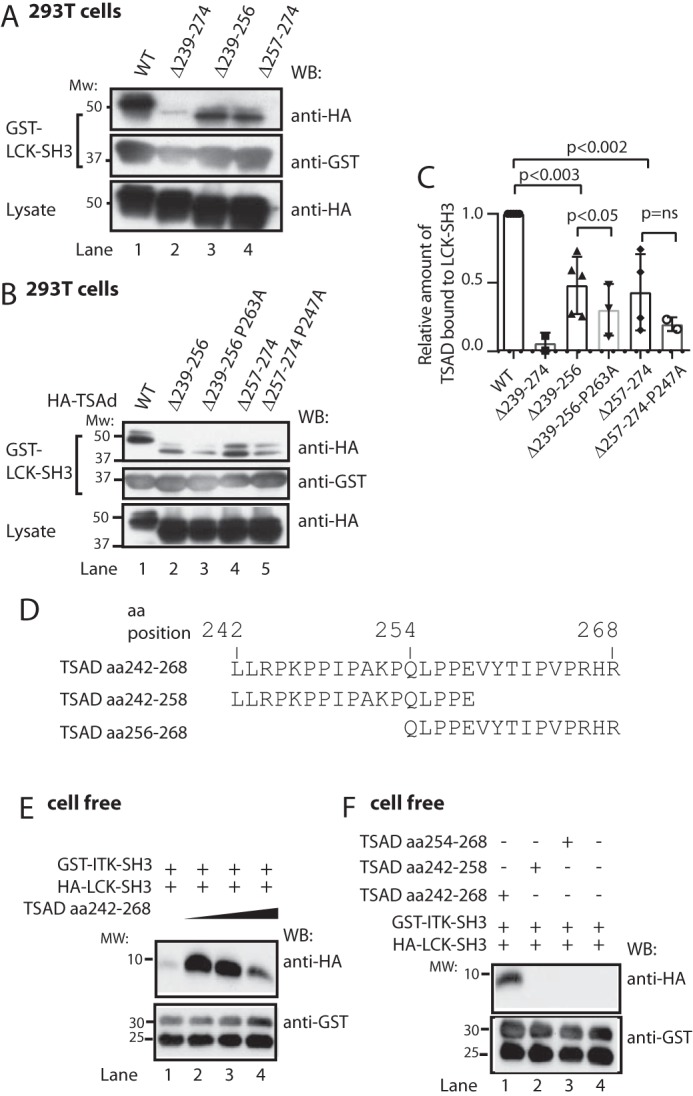
LCK SH3 and ITK SH3 may bind simultaneously to TSAD aa 242–268. A–C, LCK SH3 interacts with both TSAD aa 239–256 and aa 257–274. Additional mutation of Pro247 or Pro263 further reduces binding. A and B, pulldown experiment in 293T cells using the same TSAD constructs as in Fig. 2, E and G, respectively. Pulled down proteins and lysates were probed with the indicated antibodies. C, the graph represents ImageJ analysis of data shown in A and B (n = 3, mean ± S.D. (error bars)). D, sequences of custom synthesized TSAD peptides aa 242–268, 242–256, and 254–268. E, in vitro pulldown experiment using a 10 μm concentration of each SH3 domain and increasing amounts of TSAD aa 242–268 (10, 20, and 40 μm) in a total volume of 100 μl. The GST-ITK SH3 domain was added while attached to glutathione SepharoseTM beads. To eliminate bead loss, a 4-fold amount of GST-glutathione SepharoseTM beads was added to the mixture, as is evidenced from the bottom panel. F, in vitro pulldown experiment performed as in D, using a 10 μm concentration of each SH3 domain, and a 10 μm concentration of the indicated TSAD peptides. WB, Western blot.
