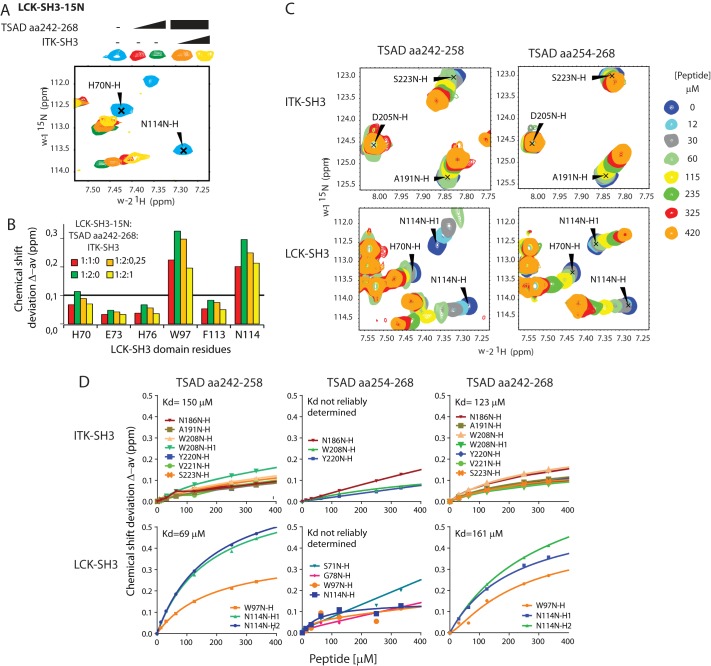
Figure 6.
ITK SH3 competes with LCK SH3 for binding to TSAD aa 242–268. A, chemical shift deviations of selected residues in HSQC titration experiments performed with 15N-labeled LCK SH3 (initially 0.25 mm) in the presence of increasing concentration of TSAD aa 242–268 (0.25 mm (red) and 0.5 mm (green)) followed by increasing amounts of nonlabeled ITK SH3 (orange, yellow). B, chemical shift deviations of selected LCK residues from the HSQC experiment depicted in A (at 1:2:0.25, concentrations of LCK SH3, TSAD peptide, and ITK SH3 were 0.2, 0.4, and 0.04 mm respectively, whereas at 1:2:1, the corresponding concentrations were 0.1, 0.2, and 0.1 mm). C, chemical shift deviations of selected residues in HSQC titration experiments performed with 15N-labeled LCK SH3 and ITK SH3 added to the same NMR tube in the presence of increasing concentration of TSAD aa 242–258 or TSAD aa 254–268 peptide. D, titration curves for the indicated peptides based on HSQC shifts of selected amino acid signals from the indicated SH3 domains.