Figure 8.
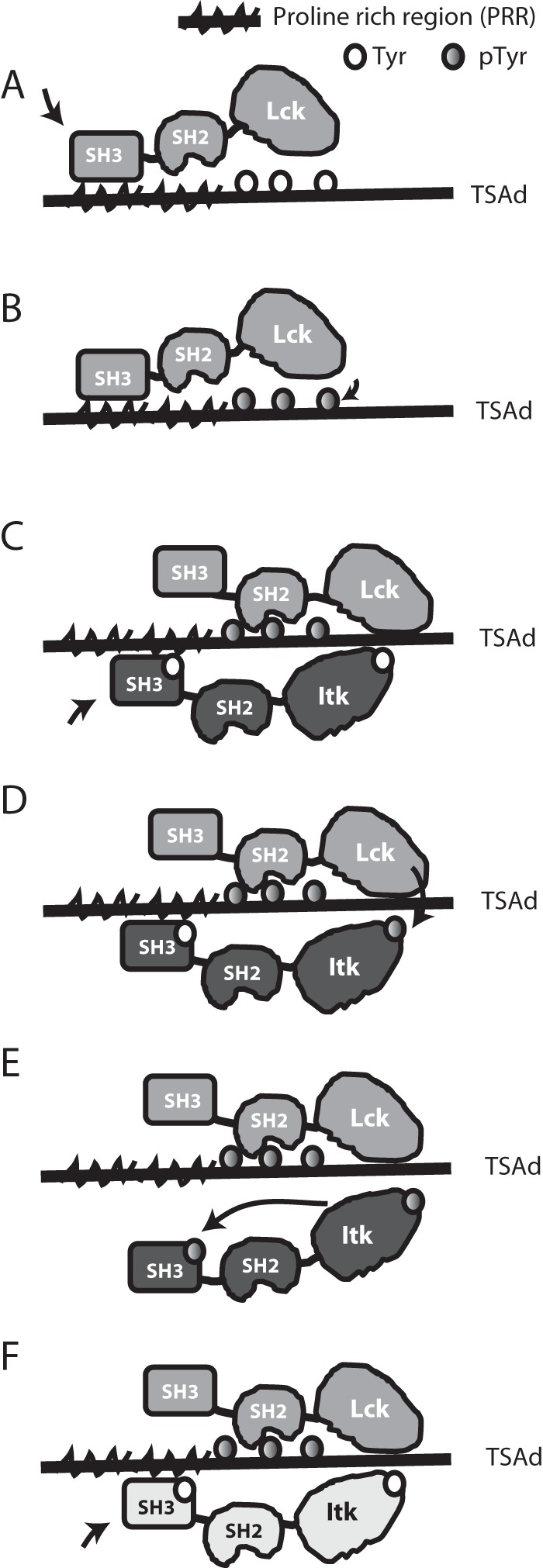
Schematic representation of TSAD-ITK-LCK interactions and their putative sequence. A, open LCK binds to TSAD PRR and becomes activated. B, active LCK phosphorylates the three TSAD C-terminal tyrosines. C, the LCK SH2 domain binds to TSAD pTyr, allowing LCK SH3 to detach from TSAD PRR. ITK binds to TSAD PRR. D, active LCK phosphorylates ITK Tyr511. E, active ITK pTyr511 autophosphorylates ITK Tyr180. ITK pTyr180 does not bind to TSAD PRR. F, active LCK remains bound to TSAD pTyr. Next, the ITK molecule (indicated in a different color) binds to TSAD PRR, and the process starts over from D.
