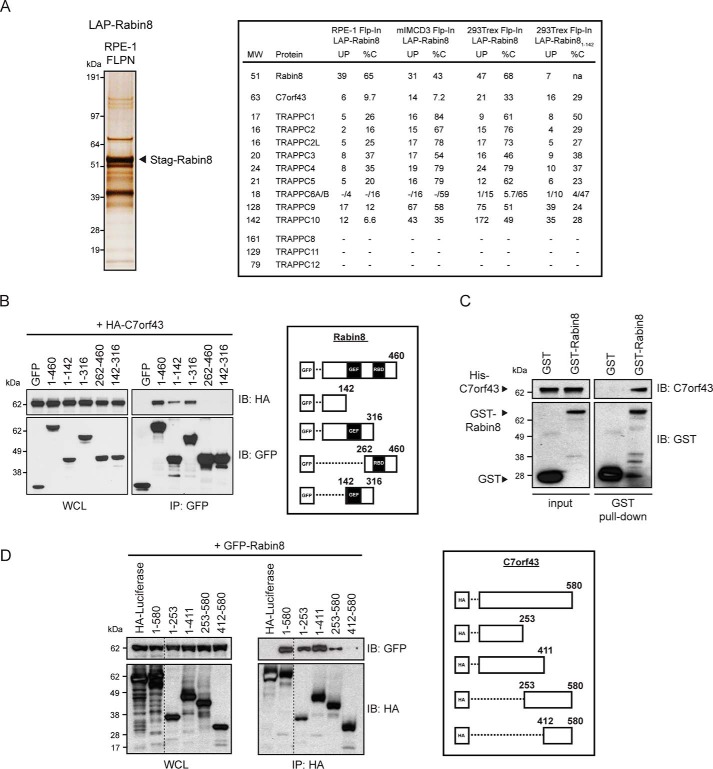
Figure 1.
C7orf43 binds to the N-terminal region of Rabin8. A, left, silver stain of SDS-polyacrylamide gel (4–12% gradient) of LAP-tagged Rabin8 purified by TAP (anti-GFP antibodies, followed by S-tag beads) from RPE-1 Flp-In stably expressing cells. 14 equally spaced gel slices were cut and analyzed by LC-MS/MS from a single experiment. Right, full-length Rabin8– and Rabin81–142–associated proteins and TRAPPCII components and C7orf43 had high-percentage peptide coverage in gel slices analyzed by LC-MS/MS corresponding to their predicted molecular weight from RPE-1 and 293Trex cells. Shown is MS analysis of full-length Rabin8 from mIMCD3 and HEK293Trex cells and Rabin81–142 from HEK293Trex from Ref. 9. UP, unique peptides; %C, percentage of amino acid coverage from peptides identified. B, domain mapping of Rabin8 for C7orf43 binding. Left, GFP antibody immunoprecipitation (IP) of GFP-Rabin8 WT and truncated fragments with HA-C7orf43 co-expressed in HEK293 cells for 48 h. Blots were probed with HA and GFP antibodies. Representative results from four independent experiments are shown. Right, schematic representation of GFP-Rabin8 full-length and deletion constructs used in immunoprecipitation. C, in vitro binding assay pull-down of recombinant His-tagged C7orf43 by GST and GST-Rabin8. Representative results from two independent experiments are shown. D, domain mapping of C7orf43 binding to Rabin8. Left, HA-beads were used to immunoprecipitate GFP-Rabin8 using HA-tagged C7orf43 full-length and deletion fragments. Representative results from two independent experiments are shown. Right, schematic representation of HA-C7orf43 full-length and deletion constructs used.