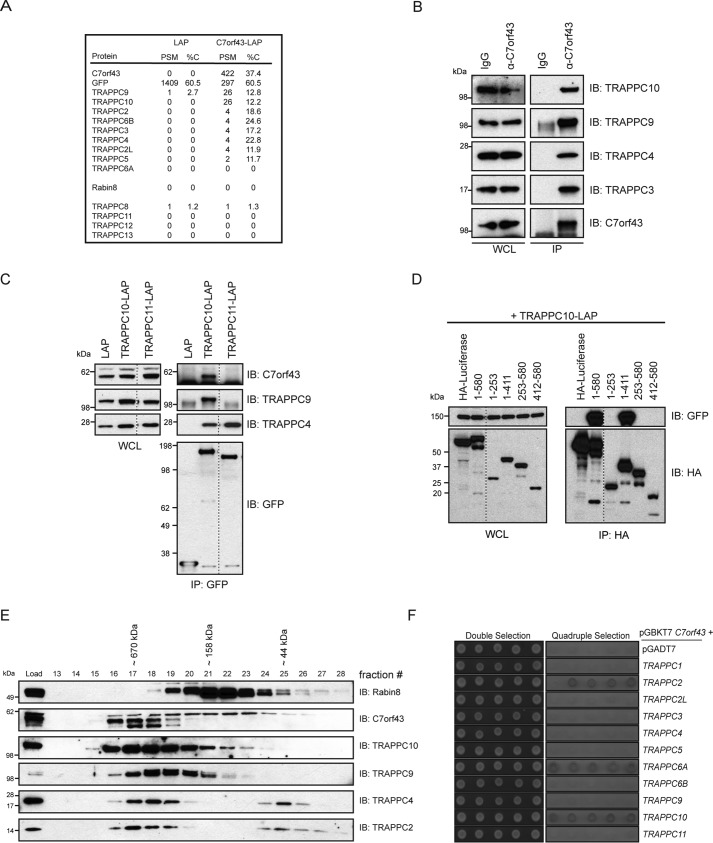
Figure 4.
C7orf43 specifically associates with the TRAPPII complex. A, PSM and percent coverage (%C) values from MS analysis of C7orf43-binding proteins immunoprecipitated with a GFP antibody from HEK293 cells transiently expressing either LAP alone or C7orf43-LAP from a single experiment. B, immunoblot (IB) showing immunoprecipitation of endogenous C7orf43 from HEK293 cells with either IgG control or C7orf43 antibody. The blot was probed with antibodies against TRAPPC proteins as indicated on the right. Representative results from two independent experiments are shown. C, immunoprecipitation of LAP-tagged proteins from HEK293 cells transfected with LAP, TRAPPC10-LAP, or TRAPPC11-LAP is shown. GFP, C7orf43, TRAPPC9, and TRAPPC4 antibodies were used for immunoblotting. Representative results from two independent experiments are shown. D, domain mapping of HA-C7orf43 for TRAPPC10-LAP binding as performed in Fig. 1D. A representative blot from three independent experiments is shown. E, immunoblotting of size-exclusion chromatography on HEK293 cell lysate using Rabin8, C7orf43, and TRAPPC antibodies. Representative results from three independent experiments are shown. F, yeast two-hybrid analysis of the GAL4 DNA-binding domain (BD) fused to C7orf43 co-transformed with GAL4 activating domain (AD) control or TRAPPC fusions. Control double selection (leucine and tryptophan) and quadruple selection (leucine, tryptophan, histidine, and adenine) are shown. Five independent colonies are shown, and the results are representative from two or three independent experiments.