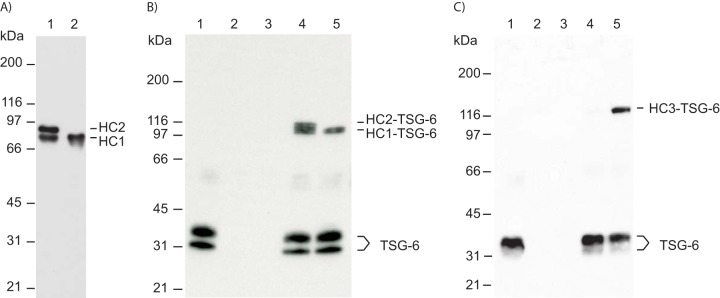
Figure 6.
C-terminally truncated HC2 maintains the ability to induce HC–TSG-6 complex formation. A, IαI was pretreated with ADAMTS-5 to release all HC2. IαI (lane 1) and IαI treated with ADAMTS-5 (lane 2) were incubated at high pH to hydrolyze the ester in the PGP cross-link. The samples were analyzed by SDS-PAGE and visualized by immunoblotting using an anti-HC1+2 antibody. After ADAMTS-5 treatment, no full-length HC2 was present (lane 2). B, TSG-6 (seen alone in lane 1) was incubated with either IαI (lane 4) or IαI pretreated with ADAMTS-5 (lane 5). The samples were analyzed by SDS-PAGE and immunoblotting using an anti-TSG-6 antibody. IαI alone and IαI pretreated with ADAMTS-5 are shown in lanes 2 and 3, respectively. Prior to ADAMTS-5 treatment, both HC1 and HC2 form a complex with TSG-6 (lane 4). Significantly, following ADAMTS-5 treatment, cleaved HC2 remains able to catalyze the formation of the HC1–TSG-6 complex (lane 5). C, to verify that cleaved HC2 is able to mediate HC–TSG-6 complex formation, cleaved HC2 was isolated after ADAMTS-5 treatment with IαI. Purified HC2 was used to evaluate complex formation between TSG-6 and HC3. TSG-6 (seen alone in lane 1) was incubated with PαI (lane 4) or PαI and purified cleaved HC2 (lane 5). The samples were analyzed by SDS-PAGE and immunoblotting using an anti-TSG-6 antibody. It is only in the presence of HC2 that TSG-6 and HC3 from PαI form the HC3–TSG-6 complex. PαI and purified HC2 are shown in lanes 2 and 3, respectively. Because complex formation between TSG-6 and HC3 is HC2-dependent, these data show that the ADAMTS-5–released HC2 is still functional.