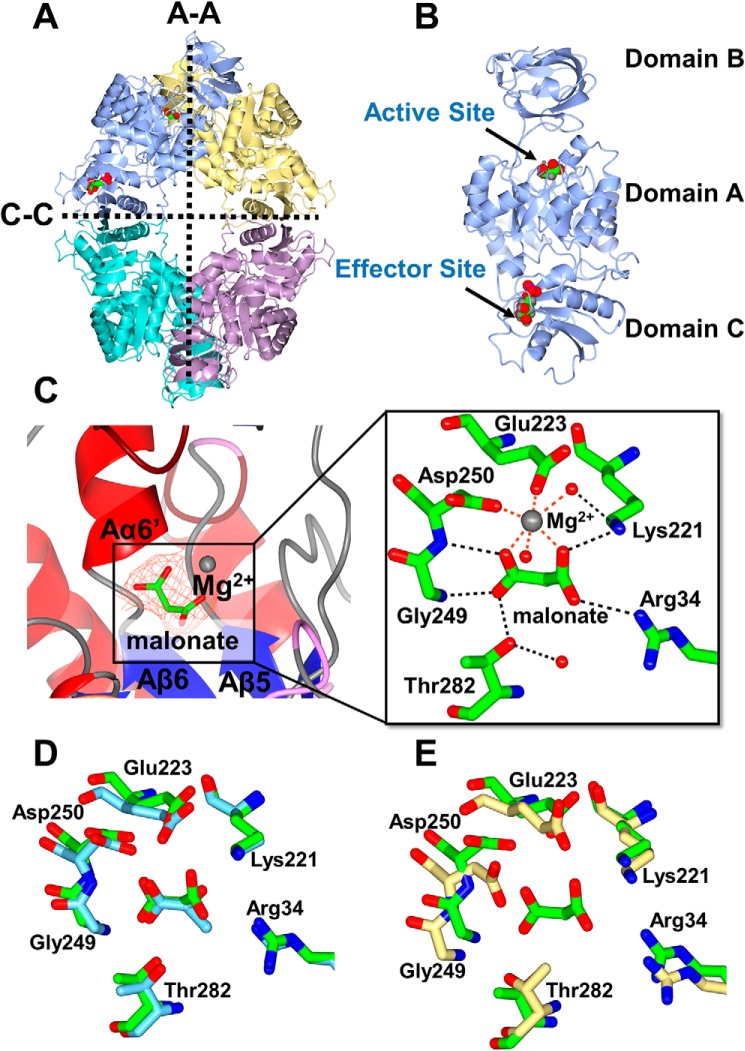
Figure 3.
X-ray crystal structure of PykA. A, the PykA homotetramer. The A-A and C-C interfaces are shown. Substrate and G6P-binding sites are indicated in the top left protomer. B, domain organization of a PykA protomer showing malonate bound in the active site and G6P bound in the allosteric site. C, close-up of the PykA active site. The network of interactions involved in holding the malonate-Mg2+ (orange mesh, Fo − Fc map contoured at 3σ) and water molecules are shown. D, superposition of the side chains involved in malonate binding in PykA (green) and pyruvate binding in rabbit muscle PK (PDB 1F3W, light blue). E, superposition of the side chains involved in malonate binding in P. aeruginosa PykA (green) with side chains present in the active site of E. coli PykF (PDB 1PKY, yellow).