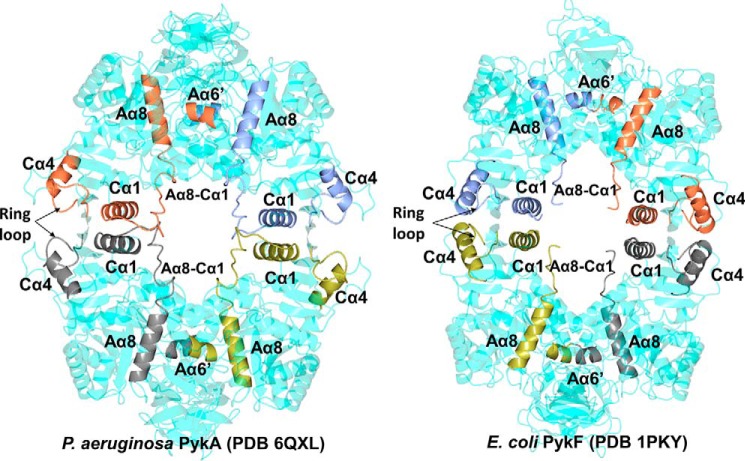
Figure 7.
Spatial disposition of key secondary structural elements in P. aeruginosa PykA and E. coli PykF. Note how the movement of the ring loop in PykA leads to disruption of the Cα4-Cα4 interaction and concomitant outward movement of Cα1. This, in turn, tugs on the Aα8-Cα1 loop, engaging it with the C-C interface. The resulting movement of Aα8 directly or indirectly affects the A-A interface by forming new interactions with Aα6-Aα6′. Given that Aα6′ forms part of the active site, this set of interactions provides a plausible mechanism by which G6P binding to the allosteric pocket in the structure can lead to altered catalysis.