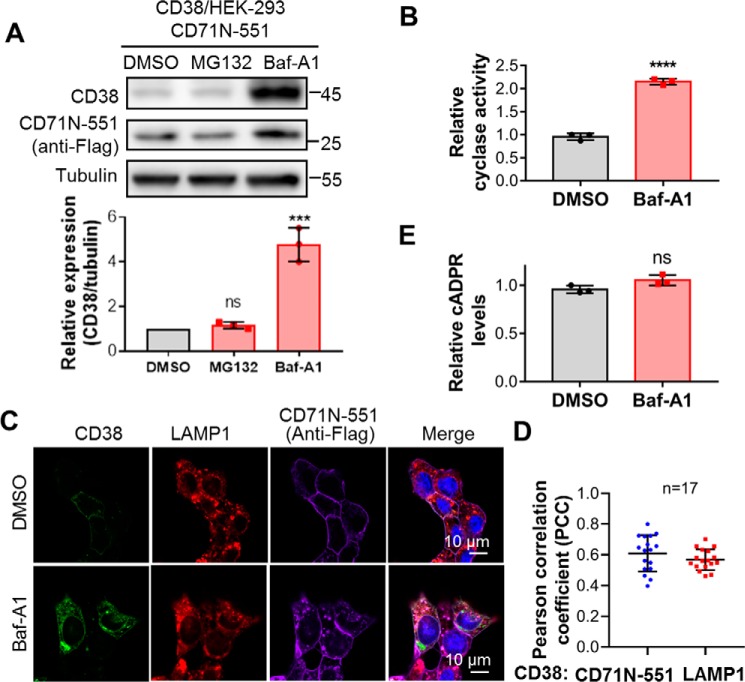
Figure 5.
CD38 was degraded in lysosomes, and lysosomal CD38 did not contribute to cADPR production. A, HEK293 cells coexpressing CD38 and CD71N-551 were treated with 5 μm MG132 for 6 h or 0.4 μm Baf-A1 for 15 h, and the cell lysates were analyzed by Western blotting with the indicated antibodies. The band intensities of CD38 were normalized by those of tubulin, plotted, and statistically analyzed by GraphPad software. B, the same cells as in A were treated with 0.4 μm Baf-A1 or DMSO as a vehicle control for 15 h, and the cell lysates were subjected to an NGD assay. The activities were normalized with those from the DMSO group. C, the same cells as in A were treated with Baf-A1 or DMSO as a control and stained with the indicated antibodies, followed by confocal imaging. Green, CD38; red, LAMP1 as an indicator of lysosomes; purple, anti-FLAG for CD71N-551; blue, DAPI for the nucleus. D, PCCs were calculated by overlapping the signals of CD38 with those of CD71N-551 (blue dots) or LAMP1 (red squares) in the presence of Baf-A1 from the experiments in C. E, the cellular cADPR contents were measured after Baf-A1 treatment. All experiments were repeated at least three times (mean ± SD; n = 3; Student's t test; ***, p < 0.001; ****, p < 0.0001; ns, not significant).