Figure 7.
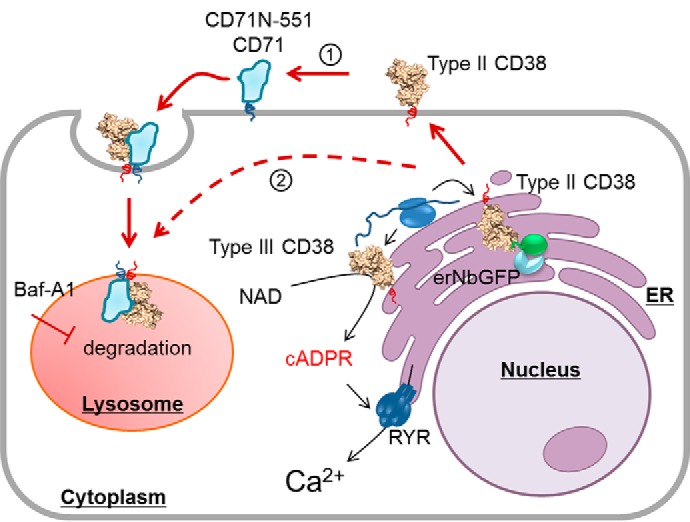
A working model of CD38 signaling. Type II CD38 is synthesized in the ER and sorted to the plasma membrane, where it interacts with the endogenous or chimeric CD71 (1). The interaction induces endocytosis, and more than 80% of CD38 is degraded in the lysosomes. Possible aberrant protein sorting (2) may also deliver type II CD38 directly to the lysosomes. The cellular levels of cADPR, however, remains unchanged. Retention of type II CD38, either in the lysosomes via Baf-A1 treatment or in the ER by expressing an ER retention protein for CD38, erNbGFP, greatly increases the type II CD38 levels in the cells but does not increase cellular cADPR levels. The results indicate that type II CD38 contributes minimally to cellular cADPR levels. This is consistent with our previously published findings documenting that it is mainly type III CD38, and not type II, that catalyzes the biosynthesis of cADPR and mediates Ca2+ signaling.
