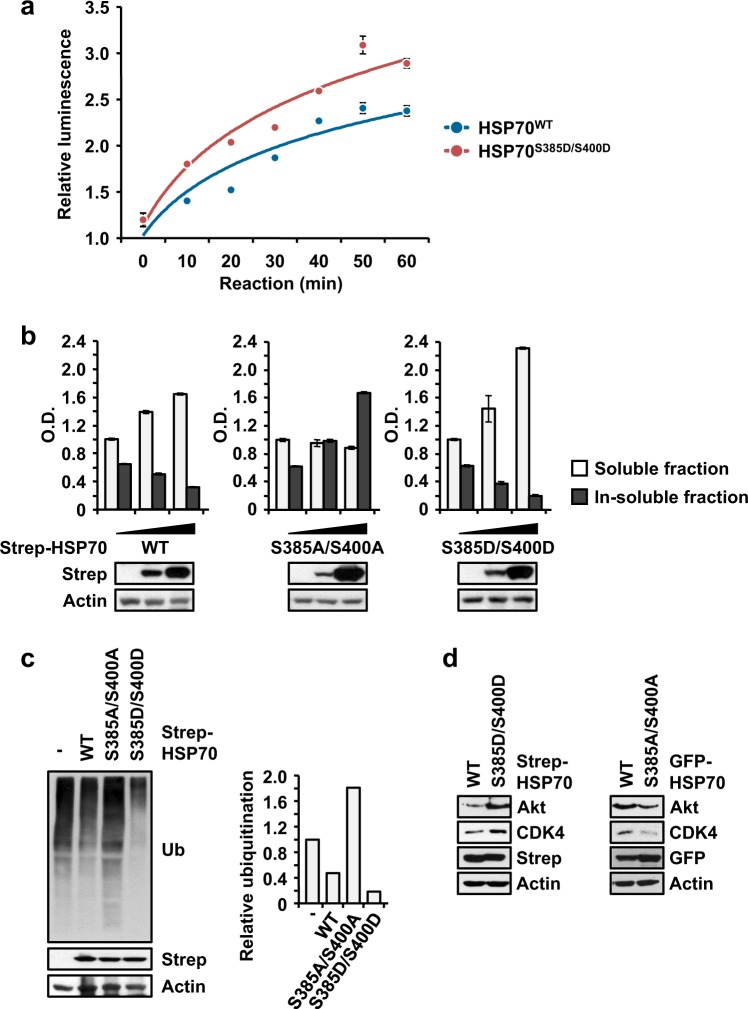
Fig. 3. Significance of HSP70 phosphorylation for protein folding.
a Refolding activity of phosphorylated HSP70. Purified wild type (WT) and double phospho-mimetic S385D/S400D HSP70 proteins were subjected to a refolding assay. The experiments were independently repeated three times, with the error bars denoting the S.D. b Determination of soluble and insoluble protein fractions following the phosphorylation of HSP70. Strep-HSP70 wild type (WT) and S385A/S400A and S385D/S400D mutant proteins were expressed in 293T cells. The soluble and insoluble protein fractions from whole cell lysates were separated and subjected to SDS-PAGE and immunoblotting using an anti-Strep antibody. Actin was used as a loading control. c Determination of ubiquitinated proteins via the phosphorylation of HSP70. The same cells as described above were treated with MG-132. The amounts of ubiquitinated proteins were determined by immunoblotting using a specific antibody against ubiquitin (Ub). The quantitated degree of ubiquitination is shown in the bar graph on the right. d The indicated HSP70 proteins were introduced into 293T cells, and the endogenous levels of Akt and CDK4 were evaluated by Western blotting using specific antibodies