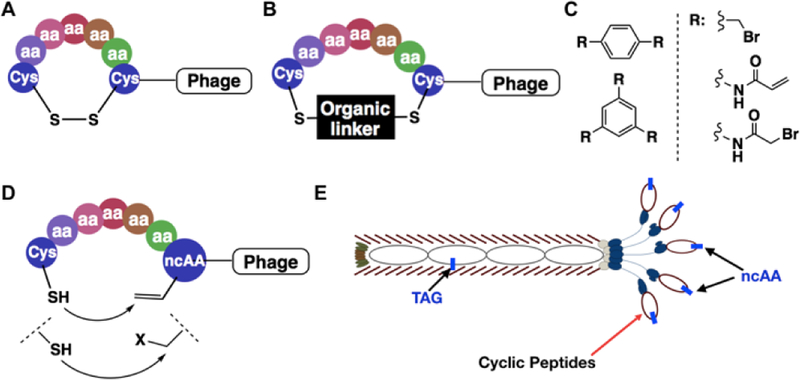
Figure 1.
Representative, existing and proposed cyclization strategies for phage-displayed peptides. (A) Cyclization through the disulfide bond between cysteines; (B) Cyclization through covalent conjugation of cysteines with organic linkers; (C) Representative organic linkers used for cysteine conjugation to generate mono- and bicyclic peptides; (D) A proposed proximity-driven cyclization between a cysteine and an electrophilic noncanonical amino acid (ncAA); (E) An amber suppression-based approach to link the phenotypic ncAA with the genotypic TAG mutation. The production of a phage with a TAG mutation at the coding region of its displayed peptide is produced in E. coli cells that harbour an evolved aminoacyl-tRNA synthetase and amber suppressing tRNA for the genetic incorporation of the designated ncAA.