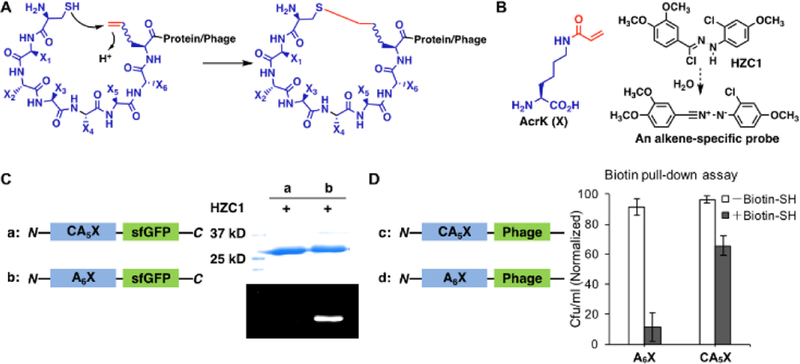
Figure 2.
Cyclization of phage-displayed peptides through Michael addition between a cysteine and a genetically incorporated Nε-acryloyl-lysine (AcrK). (A) A diagram that illustrates proximity-driven peptide cyclization between a cysteine and an electron deficient ncAA; (B) The structure of AcrK and HZC1 whose dissociation product in water is a nitrilimine that reacts selectively with an acrylamide to show intense blue fluorescence; (C) Two superfolder green fluorescent protein (sfGFP) derivatives, one with a N-terminal CA5X peptide and the other with a N-terminal A6X peptide, and their fluorescent labeling with HZC1. X denotes AcrK. Proteins were denatured first and then analyzed by SDS-PAGE. In-gel fluorescence was recorded at the blue region with the excitation at 365 nm; (D) Two phage derivatives, one with a N-terminal CA5X peptide and the other with a N-terminal A6X peptide, and their titer results after reaction with the N-biotinyl-cysteamine probe and then capturing by streptavidin beads. Uncaptured phages were analyzed in the titer assay.