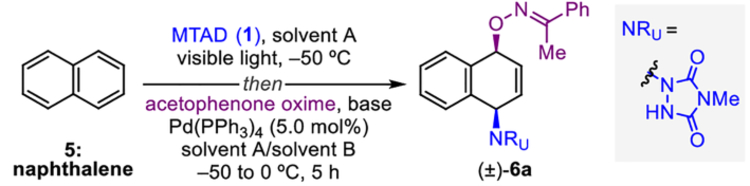
Table 1.
Optimization of reaction conditions.[a]
 | ||||
|---|---|---|---|---|
| entry | solvent A | solvent B | base | yield (%)[b] |
| 1 | CH2Cl2 | THF | KOtBu | 5 |
| 2 | CH2Cl2 | THF | LHMDS | 35 |
| 3 | CH2Cl2 | THF | nBuLi | 42 |
| 4 | Acetone | THF | nBuLi | 21 |
| 5 | EtOAc | THF | nBuLi | 10 |
| 6 | EtCN | THF | nBuLi | 52 |
| 7 | EtCN | Et2O | nBuLi | 43 |
| 8 | EtCN | PhMe | nBuLi | 71 (70) |
Reaction conditions: naphthalene (5, 1.0 mmol, 2.0 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), solvent A (0.1 M), visible light, −50 °C; then addition of solution of acetophenone oxime (1.0 mmol, 2.0 eq.) and base (0.95 mmol, 1.9 eq.) in solvent B; and Pd(PPh3)4 (5.0 mol%), −50 to 0 °C, 5 h.
Determined by 1H NMR analysis relative to the internal standard. Isolated yield shown in parentheses. THF=tetrahydrofuran, LHMDS=lithium bis(trimethylsilyl)amide
