Table 4.
Enantioselective Pd-catalyzed dearomative syn-1,4-oxyamination.[a]
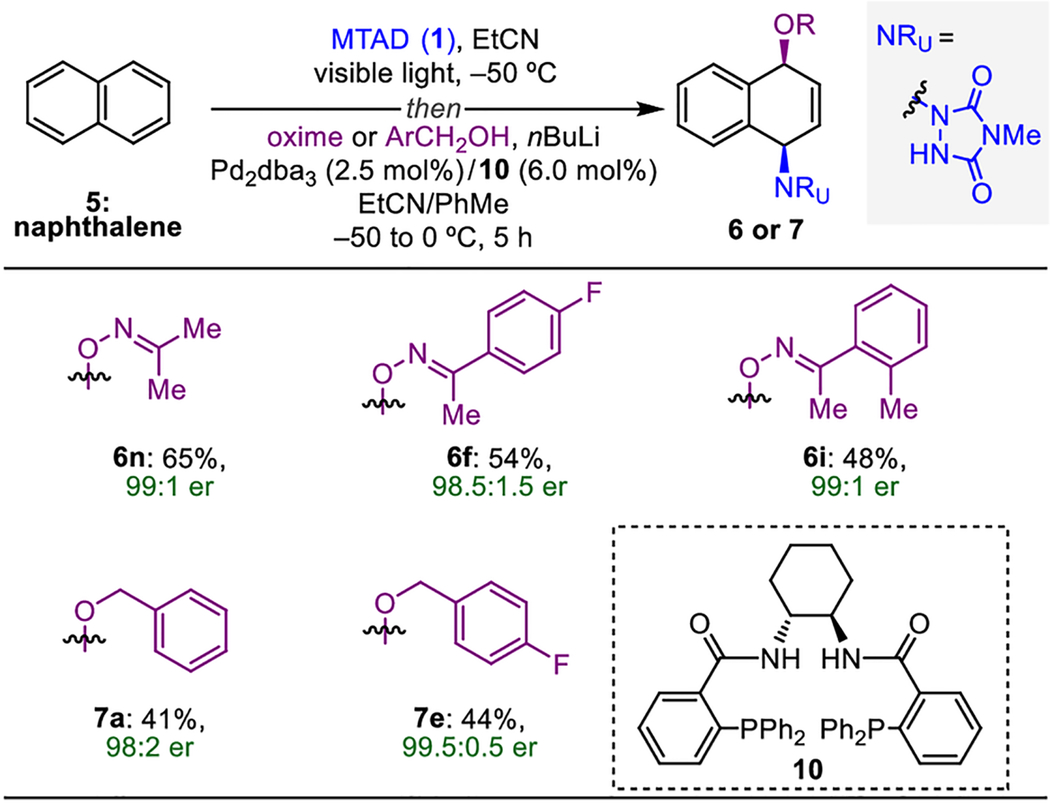
|
Reaction conditions: naphthalene (5, 1.0 mmol, 2.0 eq.), MTAD (1, 0.5 mmol, 1.0 eq.), EtCN (0.1 M), visible light, −50 °C; then addition of solution of oxime or ArCH2OH (1.0 mmol, 2.0 eq.) and nBuLi (0.95 mmol, 1.9 eq.) in PhMe; and Pd2dba3 (2,5 mol%), ligand 10 (6.0 mol%), −50 to 0 °C, 5 h. Yields of isolated product after column chromatography. Enantiomeric excess determined by HPLC analysis on a chiral stationary phase. dba=dibenzylideneacetone.
