Fig. 1. Characterization of GelMA/C bioink and resultant hydrogel.
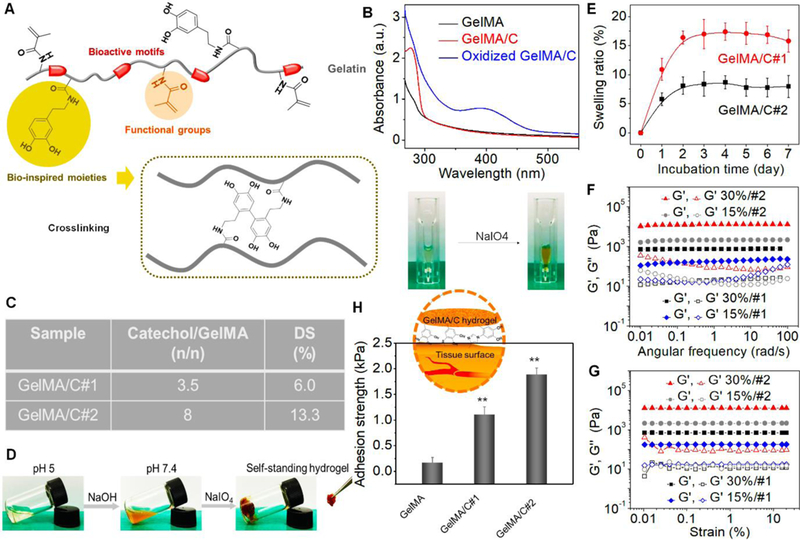
(A), Schematics of a GelMA/C copolymer structure and its oxidative crosslinking process, highlighting the key functional groups. (B), UV-Vis spectra of the GelMA/C solution (red line) that underwent oxidation (blue line), a GelMA solution serves as the control (black line). The colorless solution became brown after oxidation. (C), Degrees of substitution (DS) of catechol (GelMA/C#1and #2) determined by the standard curve method. (D), Photo images of the GelMA/C gelation process. The GelMA/C solution (colorless) at pH 5 was adjusted to pH 7.8 (brown, sol), and further crosslinked to form the self-standing hydrogel (brown, gel). (E), Structural stability of the GelMA/C hydrogel in an aqueous environment through a swelling test after 7 days of incubation. (F), Dynamic mechanical analysis of the GelMA/C hydrogels. Storage moduli (G’) and loss moduli (G”) evolution of the hydrogels with different DS and concentration (different colors) was measured in a frequency sweep mode. Filled symbols are storage moduli G’, and open symbols are loss moduli G’’. (G), Modulus-strain plots of the GelMA/C hydrogels. G’ and G’’ evolutions of the hydrogels with different DS and concentration was measured. (H), Adhesion properties of the GelMA/C hydrogels tested by detachment stress of hydrogel from the mouse muscle tissues. Compared with the GelMA hydrogel without catechol group, the GelMA/C hydrogels showed a significant increase in tissue adhesion ability due to a high binding affinity to peptides and proteins of the tissues; the mean ± sd., n≥9, **P <0.01.
