Fig. 4. Hydrodynamics, vasculoactivy, and extracellular matrix deposition.
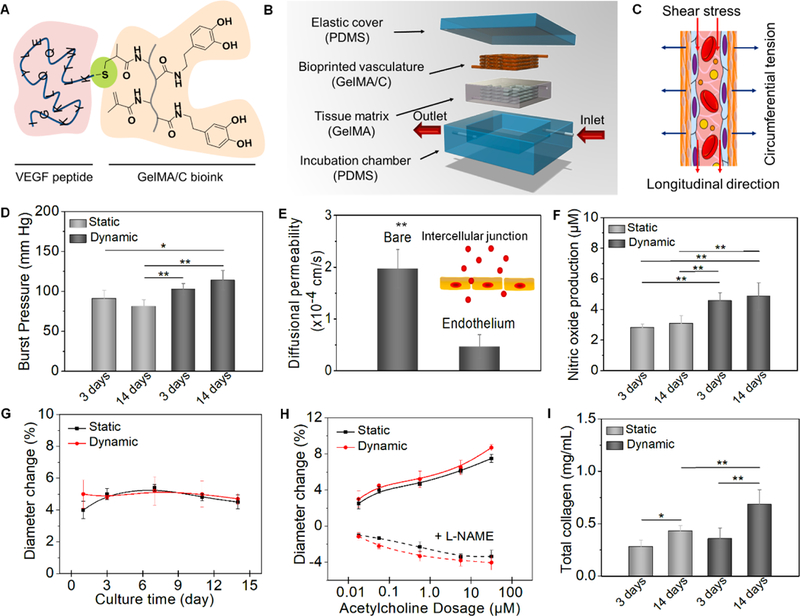
(A), schematics of chemical immobilization of VEGF peptides onto GelMA/C bioink. A Michael addition reaction between methacrylamide and thiol-functional oligopeptide was performed for ink biofunctionalization in current design. (B), an illustration of dynamic perfusion culture. The vasculature with one inlet and one outlet was embedded into a tissue matrix (GelMA hydrogel as a supporting material) and then placed into a customized PDMA chamber. (C), an illustration of the circumferential stress and shear stress during the perfusion culture. (D), the burst pressure of 3D printed vasculature constructs under static and dynamic culture; the mean ± sd., n≥9, *P <0.05, **P <0.01. (E), quantification of barrier properties confirmed by the comparison of a bare channel (without endothelial lumen) and endothelial lumen. Both contain smooth muscle cells. By mimicking native endothelial functions, vascular permeability originates from the intercellular junction; the mean ± sd., n≥9, **P <0.01. (F), nitric oxide production of 3D printed vasculature constructs under static and dynamic culture; the mean ± sd., n≥9, **P <0.01. (G), vasodilation response to 1 μM acetylcholine of 3D printed vasculature constructs under static and dynamic culture over 2 weeks; the mean ± sd., n≥6. (H), dose-dependent vasodilation response to acetylcholine of 3D printed vasculature constructs and their constriction induced by pre-treat of L-NAME under static and dynamic culture; the mean ± sd., n≥6. (I), total collagen synthesis of 3D printed vasculature constructs under static and dynamic culture; the mean ± sd., n≥9, *P <0.05, **P <0.01.
