Abstract
Background
Meningitis and encephalitis are life-threatening syndromes with high morbidity and mortality in children. Due to limitations of traditional laboratory approaches in etiological diagnosis, the rate of misdiagnoses is unacceptably high.
Methods
We retrospectively compared the potential clinical impact of the FilmArray meningitis/encephalitis (ME) panel vs. conventional cerebrospinal fluid (CSF) culture in children with central nervous system (CNS) infections. Sixty-eight pediatric patients (<18 years of age) with an initial diagnosis of meningitis or encephalitis were enrolled at 2 children’s hospital from January to October 2017.
Results
Fifteen specimens were found to be positive after CSF culture, with a positive rate of 22.1% (15/68). For the FilmArray ME panel, 26 bacteria and fungi from 25 samples were detected, and the positive rate was 36.8% (25/68). The FilmArray ME panel identified 14 pathogens in previously pathogen-negative patients.
Conclusions
This study demonstrated the capability of the FilmArray ME panel in the diagnosis of bacterial and fungal meningitis and therefore its potential use in facilitating enhanced patient care.
Keywords: Meningitis, encephalitis, rapid diagnosis, molecular diagnostic tests, FilmArray ME panel
Introduction
Meningitis and encephalitis are life-threatening syndromes in children that can be caused by bacteria, yeasts or viruses. The morbidity and mortality of these infections can be high, particularly with bacterial-fungal meningitis. In China, the incidence of acute bacterial meningitis ranges from 6.95 to 22.3 cases/10,000 children <5 years of age (1,2). It has been reported that Neisseria meningitidis (N. meningitidis), Haemophilus influenzae (H. influenzae) type b and Streptococcus pneumoniae (S. pneumoniae) are among the most prevalent pathogens in children (3,4). Prompt diagnosis and appropriate antibiotic utilization are necessary to minimize adverse outcomes.
Despite being time-consuming and having low sensitivity (particularly in patients pretreated with antibiotics), routine culture remains the gold standard for the diagnosis of bacterial and fungal meningitis (5). However, the limitations of traditional laboratory approaches lead to unnecessarily prolonged empirical antibiotic treatment and an increase in the number of hospital admissions as well as the duration of hospital stays. In addition, the lack of routine diagnostic tests for viral causes of meningitis in China also complicates the problem (6). Rapid, sensitive and comprehensive tests, such as molecular diagnostic tests, may be helpful to overcome the limitations of conventional laboratory-based diagnosis (7).
The FilmArray meningitis/encephalitis (ME) panel (BioFire Diagnostics, Utah, USA, owned by bioMérieux) uses multiplex PCR to detect 14 common pathogens, namely, Escherichia coli K1 (E. coli K1), H. influenzae, Listeria monocytogenes (L. monocytogenes), N. meningitidis, Streptococcus agalactiae (S. agalactiae), S. pneumoniae, cytomegalovirus (CMV), enterovirus (EV), herpes simplex virus 1 and 2 (HSV-1, HSV-2), human herpesvirus 6 (HHV-6), human parechovirus (HPeV), varicella zoster virus (VZV), and Cryptococcus neoformans/Cryptococcus gattii (Cr. neoformans/C. gattii). The entire process is fully automated and takes only approximately one hour to obtain the diagnostic results. The FilmArray ME panel has been increasingly used in many western countries in recent years (8,9). However, its clinical significance in diagnosing ME etiology in the Chinese population is sparse since it is rarely utilized in China. Therefore, in this study, we retrospectively compared the potential clinical impact of the FilmArray ME panel vs. conventional cerebrospinal fluid (CSF) culture in children with suspected or confirmed central nervous system (CNS) infections.
Methods
Clinical specimens
This research was a retrospective study. The study was conducted at 2 children’s hospitals, namely, Shanghai Children’s Medical Center and Zhejiang Children’s Hospital, from January 2017 to October 2017. The study was approved by the Institutional Review Board and the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2017059). Written informed consent was obtained from the parents of the participants when lumbar puncture (LP) was carried out.
Pediatric patients (<18 years old) with an initial diagnosis of meningitis or encephalitis were enrolled. Meningitis or encephalitis was defined according to the World Health Organization (WHO) workbook recommendations based on laboratory findings, symptoms, or signs. In addition, all patients were subjected to the following: (I) complete medical history and (II) full clinical examination. Patients were excluded from the study if they met the following criteria: (I) cases complicated with congenital diseases or chronic medical conditions and (II) cases in which other CNS disorders could not be excluded.
For FilmArray ME panel testing, specimens meeting the following inclusion criteria were selected: CSF specimens were collected by LP with adequate volume (>1 mL); specimens were stored at −80 °C for later testing. Duplicate specimens from the same subject were excluded.
FilmArray ME panel testing
The FilmArray ME panel testing procedure was performed at Shanghai Children’s Medical Center according to the manufacturer’s instructions. The operation was performed by independent researchers who were blinded to the diagnosis. The test consisted of automated nucleic acid extraction, reverse transcription and nucleic acid amplification. Comprehensive results were available within approximately 1 hour.
Bacterial and fungal conventional testing
Conventional bacterial and fungal testing programs, including CSF culture, blood culture, Gram strain, ink stain, physiology and biochemistry of CSF, latex agglutination test and serum virology were performed on every subject enrolled. Testing was performed at either hospital using the laboratories’ standard procedures.
PCR and sequencing to detect CSF bacterial and fungal infection
Nucleic acid was extracted from each specimen using a QIAamp DNA minikit (Qiagen, Hilden, Germany). PCR was performed using universal primers for the bacterial 16S rDNA gene and fungal 26S rDNA gene. The sequence of the primers used for PCR amplification is provided in Table S1. Pathogens were identified by analyzing DNA sequences using the BLAST tool of NCBI. The results of the PCR analysis were reported as part of the data used for the discrepancy investigation.
Table S1. Primers used in the PCR amplification.
| Primer | Forward | Reverse |
|---|---|---|
| Bacteria-16S | AGAGTTTGATCMTGGCTCAG | TACGGYTACCTTGTTACGACTT |
| Fungus-26S | GCATATCAATAAGCGGAGGAAAAG | GGTCCGTGTTTCAAGACGG |
| E.coli-mdh | TGGTTGCTAACTGGAAAGGAAT | ACGTGTATTTGAAGCATTGCTG |
| S. pneumoniae-ply | CCCACTCTTCTTGCGGTTGA | TGAGCCGTTATTTTTTCATACTG |
| S. agalactiae-cps | CAATCCTAAGTATTTTCGGTTCATT | TAGGAACATGTTCATTAACATAGC |
Discrepancy analysis
Samples with discrepant results between the CSF culture and the FilmArray ME panel were reanalyzed using a targeted PCR assay. A FilmArray ME panel result was considered true positive (TP) or true negative (TN) only when it agreed with the results from CSF culture or the comparator methods. Otherwise, the results were judged as false positive (FP) or false negative (FN).
Data analysis
Statistical analysis was performed using SPSS ver. 22. Demographic data are presented as descriptive statistics. The agreement between assays was measured using the kappa statistic. The overall percentage of agreement (OPA) was calculated as previously described (10). Briefly, OPA was calculated as [(TP+TN)/(TP+TN+FP+FN)] ×100%. The sensitivity and specificity were compared for all tests.
Results
Patient population and clinical feature
A total of 223 patients were screened, for whom 68 CSF samples met the inclusion criteria between January and October 2017 (Figure 1). The average age of the patients was 2.76 years (range, 3 days to 12 years), and the male/female ratio was 2.09 (46:22). There were 21 (30.9%) newborns (ages: 1–28 days), 14 (20.6%) infants (ages: 2–12 months), 23 (33.8%) preschoolers (ages: 2–6 years) and 10 (14.7%) school-aged children (ages: 7–18 years). A total of 50 patients (73.5%) in the study had received antibiotic treatment (ceftazidime, meropenem, penicillin, etc.) before LP. The correlation between the number of days that each patient was on antibiotics before LP and the CSF culture and FA ME panel results is illustrated in Figure 2. Thirty-five patients (51.5%) were diagnosed with bacterial meningitis, 16 patients (23.5%) with viral encephalitis and 1 patient (1.5%) with cryptococcal meningoencephalitis. Their general characteristics are presented in Table 1.
Figure 1.

The enrollment process of patients with meningitis or encephalitis.
Figure 2.
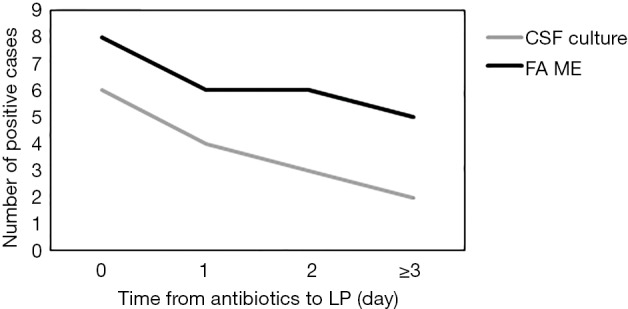
The correlation between the number of days that each patient was on antibiotics before lumbar puncture and the CSF culture and FA ME panel results. CSF, cerebrospinal fluid; FA ME, FilmArray meningitis/encephalitis.
Table 1. General characteristics of the patients.
| Characteristic | Cases (%) |
|---|---|
| Total | 68 (100.0) |
| Age | |
| <1 month | 21 (30.9) |
| 1–11 months | 14 (20.6) |
| 2–6 years | 23 (33.8) |
| 7–18 years | 10 (14.7) |
| Gender | |
| Male | 46 (67.6) |
| Female | 22 (32.4) |
| Antibiotic use before LP | |
| Yes | 50 (73.5) |
| No | 18 (26.5) |
| Clinical diagnosis | |
| Bacterial meningitis | 35 (51.5) |
| Viral encephalitis | 16 (23.5) |
| Cryptococcal meningoencephalitis | 1 (1.5) |
| Others | 16 (23.5) |
LP, lumbar puncture.
Pathogens detected in pediatric patients
Fifteen specimens were found to be positive after CSF culture, with a positive rate of 22.1% (15/68). For the FilmArray ME panel, 26 bacteria and fungi from 25 samples were detected, and the positive rate was 36.8% (25/68) (Table 2). The FilmArray ME panel identified 14 pathogens in previously CSF culture-negative patients, while CSF culture identified pathogens in 4 of the 30 FilmArray ME panel-negative samples (Table 3). The most prevalent in the FilmArray ME panel detection were E. coli K1 (8/68, 11.8%), S. pneumoniae (8/68, 11.8%) and S. agalactiae (6/68 8.8%). L. monocytogenes and N. meningitidis were detected in 1 (1/68, 1.5%) sample each, and C. neoformans/C. gattii was detected in 2 (2/68, 2.9%) samples.
Table 2. Distribution of bacteria and yeast identified by the FilmArray ME (FA ME) panel and CSF culture.
| Pathogen identified | FA ME panel | CSF culture | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. detected | No. of positive detections by age group | No. detected | No. of positive detections by age group | ||||||||
| <1 mo | 1–11 mo | 2–6 yr | 7–18 yr | <1 m | 1–11 m | 2–6 yr | 7–18 yr | ||||
| E. coli K1 | 8 | 5 | 1 | 2 | 0 | 5 | 4 | 0 | 1 | 0 | |
| H. influenzae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| L. monocytogenes | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| N. meningitidis | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S. agalactiae | 6 | 4 | 2 | 0 | 0 | 3 | 2 | 1 | 0 | 0 | |
| S. pneumoniae | 8 | 0 | 2 | 6 | 0 | 5 | 0 | 0 | 5 | 0 | |
| Cr. neoformans/C. gattii | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | |
| Total | 26 | 10 | 6 | 9 | 1 | 15 | 6 | 2 | 6 | 1 | |
CSF, cerebrospinal fluid.
Table 3. Comparison of the positive and negative results in the FilmArray ME panel and comparator assays in bacteria and yeast detection.
| Pathogen identified | No. of results | Discordant results | ||||||
|---|---|---|---|---|---|---|---|---|
| C−/F− | C+/F+ | C+/F− | C−/F+ | F+/P+ | F+/P− | F−/P+ | ||
| E. coli K1 | 58 | 3 | 2 | 5 | 4 | 1 | 1 | |
| H. influenzae | 67 | 0 | 1 | 0 | NA | NA | NA | |
| L. monocytogenes | 67 | 0 | 0 | 1 | 1 | 0 | 0 | |
| N. meningitidis | 67 | 0 | 0 | 1 | 1 | 0 | 0 | |
| S. agalactiae | 62 | 3 | 0 | 3 | 2 | 1 | 0 | |
| S. pneumoniae | 59 | 4 | 1 | 4 | 3 | 1 | 0 | |
| Cr. neoformans/C. gattii | 66 | 1 | 0 | 1 | 1 | 0 | 0 | |
C, CSF culture; F, FilmArray ME panel; P, Target PCR assay; +, positive result; −, negative result. NA, not applicable or not able to calculate.
Among the FilmArray ME panel results, viral pathogens were detected in 17 samples, namely, EV (10 cases), CMV (5 cases), HSV-1 (1 case) and HHV-6 (1 case) (Figure 3). The patients’ clinical data are reported in Table S2.
Figure 3.
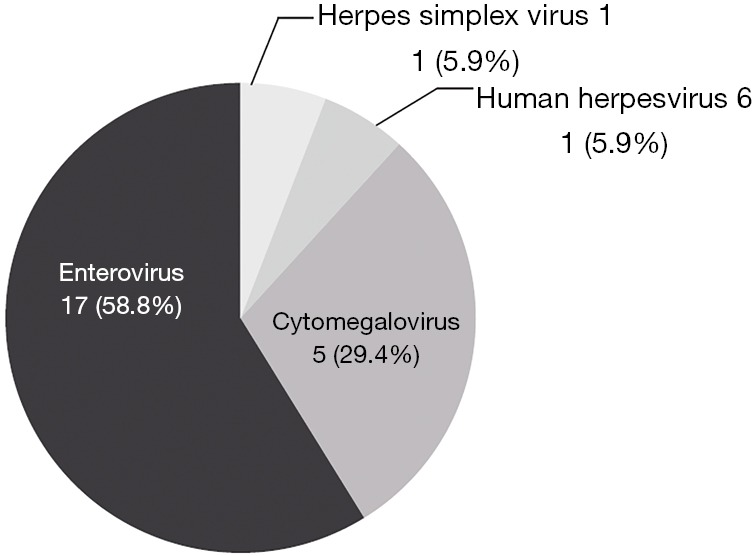
Virus detected by the FilmArray ME panel. ME, meningitis/encephalitis.
Table S2. Summary of clinical data on patients with positive viral pathogens confirmed by the FilmArray ME panel.
| Patient | Clinical diagnosis from medical records | CSF cells | CSF protein | CSF glucose | CSF chlorides | Serological test | FA ME detection |
|---|---|---|---|---|---|---|---|
| 1 | Purulent meningitis | 6929 | 3,529.9 | 2.77 | 114.7 | – | CMV |
| 2 | Viral encephalitis | 106 | 193 | 3.35 | 124.7 | – | EV |
| 3 | Viral encephalitis | 1360 | 244 | 2.61 | 124.6 | – | EV |
| 4 | Purulent meningitis | 500 | 418 | 2.85 | 120.4 | – | EV |
| 5 | Viral encephalitis | 440 | 193 | 3.33 | 122.8 | – | EV |
| 6 | Viral encephalitis | 300 | 1,273 | 1.76 | 121.8 | – | EV |
| 7 | Viral encephalitis | 61 | 1,546 | 2.3 | 127 | HSV-1 IgM(−), IgG(−) | HSV-1 |
| 8 | Purulent meningitis | 90 | 703 | 2.3 | 118 | – | EV |
| 9 | CNS infection | 112 | 1,955 | 2.1 | 120 | – | EV |
| 10 | Viral encephalitis | 0 | <100 | 3.3 | 127 | EV71 IgM(±) | EV |
| 11 | Viral encephalitis | 160 | 405 | 3.4 | 121 | – | EV |
| 12 | Viral encephalitis | 600 | 235 | 2.9 | 122 | – | EV |
| 13 | Viral encephalitis | 87 | 527 | 3.1 | 125 | – | CMV/HHV-6 |
Correlation between the FilmArray ME panel and CSF culture in detecting bacteria and yeast
In this study, a total of 476 individual FilmArray ME panel analyte tests were performed on 68 samples (for each sample, 6 bacterial and 1 fungal analyte tests were included: E. coli K1, H. influenzae, L. monocytogenes, N. meningitidis, S. pneumoniae, S. agalactiae and Cr. neoformans/C. gattii). The OPA between the FilmArray ME panel and CSF culture were 83.2% (396/476). For the individual target, the FilmArray ME panel had a relatively higher OPA than the CSF culture for the detection of bacteria and yeast, including H. influenzae, L. monocytogenes, N. meningitidis, S. agalactiae, and C. neoformans/C. gattii, while two analytes had lower sensitivities (89.7% for E. coli K1 and 92.7% for S. pneumoniae) (Table 4). Using CSF culture as the diagnostic gold standard, the sensitivity and specificity of the individual FilmArray ME panel’s components were calculated. The FilmArray ME panel demonstrated a sensitivity of 100% for 2 analytes: S. agalactiae and C. neoformans/C. gattii. The specificity was 92.1% or greater for all analytes. Comparison of FilmArray ME panel and CSF culture for E. coli K1, S. agalactiae, S. pneumoniae, and Cr. neoformans/C. gattii showed moderate agreement (0.4< kappa <0.7).
Table 4. Performance summary and characteristics of the FilmArray ME panel and CSF culture in bacteria and yeast detection.
| Pathogen identified | OPA (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Kappa |
|---|---|---|---|---|---|---|
| E. coli K1 | 89.7 | 60 | 92.1 | 37.5 | 96.7 | 0.408 |
| H. influenzae | 98.5 | 0 | 100 | 0 | 98.5 | 0 |
| L. monocytogenes | 98.5 | NA | 98.5 | 0 | 100 | 0 |
| N. meningitidis | 98.5 | NA | 98.5 | 0 | 100 | 0 |
| S. agalactiae | 95.6 | 100 | 95.4 | 50 | 100 | 0.646 |
| S. pneumoniae | 92.7 | 80 | 93.7 | 50 | 98.3 | 0.577 |
| Cr. neoformans/C. gattii | 98.5 | 100 | 98.5 | 50 | 100 | 0.66 |
OPA, overall percentage of agreement; PPV, positive predictive value; NPV, negative predictive value.
Analysis of discrepant results
The 19 samples with discrepant results are summarized in Table 5. There were 12 TP cases (E. coli K1: 4 cases; S. agalactiae: 2 cases; S. pneumoniae: 3 cases; L. monocytogenes: 1 case; N. meningitides: 1 case; C. neoformans/C. gattii: 1 case) and 3 TN cases (E. coli K1: 1 case; S. pneumoniae: 1 case; H. influenzae: 1 case) for the FilmArray ME panel results using comparator testing results (target PCR assay). The 1 FN case (E. coli K1: 1 case) and 3 FP cases (E. coli K1: 1 case; S. agalactiae: 1 case; S. pneumoniae: 1 case) were determined with no additional evidence.
Table 5. Discrepant investigation for samples with discordant results.
| Patient | FA ME detection | CSF culture | Target PCR assay | Additional analysis or supplemental testing | Clinical diagnosis from medical records | Final resolution of FA ME result |
|---|---|---|---|---|---|---|
| 1 | Negativea | E. coli | E. coli | Blood culture = E. coli | Bacterial meningitis | False negative |
| 2 | Negative | E. coli | Negative | Blood culture = E. coli | Bacterial meningitis | True negative |
| 3 | E. coli K1 | Negative | E. coli | Blood culture = E. coli | Bacterial meningitis | True positive |
| 4 | E. coli K1 | Negative | E. coli | Blood culture = negative | Bacterial meningitis | True positive |
| 5 | E. coli K1 | Negative | Negative | Blood culture = S. agalactiae | Septicemia | False positive |
| 6 | E. coli K1 | Negative | E. coli | Blood culture = E. coli | Bacterial meningitis | True positive |
| 7 | E. coli K1 | Negative | E. coli | Blood culture = E. coli | Bacterial meningitis | True positive |
| 8 | S. agalactiae | Negative | Negative | Blood culture = negative | Bacterial meningitis | False positive |
| 9 | S. agalactiae | Negative | S. agalactiae | Blood culture = negative | Bacterial meningitis | True positive |
| 10 | S. agalactiae | Negative | S. agalactiae | Blood culture = S. haemolyticus | Bacterial meningitis | True positive |
| 11 | S. pneumoniae | Negative | Negative | S. pneumoniae antibody = positive | Bacterial meningitis | False positive |
| 12 | S. pneumoniae | Negative | S. pneumoniae | S. pneumoniae antibody = positive | Bacterial meningitis | True positive |
| 13 | S. pneumoniae | Negative | S. pneumoniae | Blood culture = negative | Bacterial meningitis | True positive |
| 14 | S. pneumoniae | Negative | S. pneumoniae | Blood culture = negative | Bacterial meningitis | True positive |
| 15 | Negative | S. pneumoniae | Negative | S. pneumoniae antibody = positive | Bacterial meningitis | True negative |
| 16 | L. monocytogenes | Negative | L. monocytoge | Blood culture = L. monocytoge | CNS infectious | True positive |
| 17 | Negative | H. influenzae | Negative | Blood culture = negative | Bacterial meningitis | True negative |
| 18 | N. meningitidis | Negative | N. meningitidis | Blood culture = negative | Bacterial meningitis | True positive |
| 19 | Cr. neoformans/C. gattii | Negative | Cr. Neoformans/C.gattii | Blood culture = negative | Cryptococcal meningoencephalitis | True positive |
a, for E. coli, only the K1 capsular type is detected by the FilmArray ME panel.
Discussion
As a major health problem in newborn infants and children worldwide, meningitis and encephalitis require early diagnosis and aggressive therapy (11,12). However, similarities exist in patients with different pathogen infections in terms of clinical manifestations, making it difficult to diagnose meningitis and encephalitis with atypical clinical symptoms and signs. Due to the lack of rapid and reliable laboratory tests in etiological diagnosis, the rate of erroneous diagnosis is unacceptably high. Until recently, the etiology was unknown for approximately 50% of cases (13,14), leading to a delay in the initiation of optimal treatment. Novel and fast molecular techniques help identify etiologies, prevent the use of unnecessary antibiotics and shorten the length of hospital stays (15). The BioFire FilmArray ME panel provides a comprehensive panel testing for 14 CNS pathogens simultaneously using a minimal amount of CSF with a rapid turn-around time (10). More recently, the use of the FilmArray ME panel for detecting pathogens has been reported to improve laboratory diagnosis (16,17). To our knowledge, this study is the first report of the performance of the FilmArray ME panel in China, where we evaluated the potential clinical benefits in testing for various pathogens.
In our study, demographic data analysis of patients revealed that there were more males than females (67.6% vs. 32.4%). This finding agreed with a study by Qazi et al., which showed that males were more significantly affected by bacterial meningitis than females (80% vs. 20%) (18). This difference may signify male dominance and sex discrimination in East Asia. A total of 51.5% (n=35) of our patients were below 1 year of age, showing that meningitis is more likely to occur in younger children than in older children. A study by Seth et al. confirmed that meningitis is most strongly and consistently associated with a young age, in which the majority of patients (76%) were infants <12 months old (19).
The definitive diagnosis of bacterial meningitis has been historically based on culture, which has a sensitivity of ≤80% (20). As a gold standard for the diagnosis of meningitis, CSF culture was therefore used as the comparator assay in the present research. Previous studies have shown that CSF culture was positive in only 10% of antibiotic-pretreated patients in developing countries (21). Afifi et al. also found low rates of culture-positive CSF samples (8%) in suspected cases of bacterial meningitis (22). In our study, 50 patients (73.5%) had received antibiotic treatment before a LP was performed. Among these patients, only 10 (14.7%) samples were positive in CSF culture, while 18 (26.4%) samples were positive when detected with the FilmArray ME panel. In addition, the positive rate was influenced by the therapeutic time of antibiotics before LP. The detection number was lower with a longer use time of antibiotics in both methods. However, the FilmArray ME panel had relatively higher sensitivity than CSF culture when the use time of antibiotics was more than 1 day. Among 51 culture-negative CSF specimens, 14 were positive in the FilmArray ME panel detection. Wootton et al. confirmed that the FilmArray ME panel could enhance pathogen identification in CNS-infected patients with a negative Gram stain, and the panel detected pathogens not previously identified in 11 (22.9%) of 48 patients (17). These findings show that the FilmArray ME panel can provide enhanced diagnosis in culture or Gram stain negative CSF specimens, especially after the administration of antimicrobial therapy.
Using FilmArray ME panel detection, this study identified bacteria and yeast in 25/68 patients (36.8%). E. coli K1 and S. pneumoniae were the most common organisms detected, followed by S. agalactiae, C. Neoformans, L. monocytogenes and N. meningitidis. The FilmArray ME panel had a higher sensitivity than CSF culture in detecting almost all bacteria and yeast except H. influenzae. Furthermore, the association between age and pathogens was analyzed. In our study, E. coli K1 and S. agalactiae were the predominant pathogens inducing neonatal bacterial meningitis. The same result was reported by Arora et al., namely, that the FilmArray ME panel enhanced the identification of group B Streptococcus and E. coli in young infants with meningitis (16). Other studies in Australia, London and Canada also documented similar patterns, with S. agalactiae and E. coli being the major etiological agents for neonatal bacterial meningitis infection (23-25). S. pneumoniae was the predominant pathogen isolated in the 1–6 years age group, and this finding was consistent with a Korean study in which S. pneumoniae was the most detected etiologic pathogen beyond the neonatal period (26). Although mixed CNS infections are not uncommon in children, especially in immunocompromised individuals, infections with two or more pathogens are not easily detected by conventional methods. Hence, the biological significance of dual infections is currently not well understood. The FilmArray ME panel has the significant benefit of being able to identify coinfections. Five cases of dual infections were detected in our study, namely, a case of mixed bacterial-bacterial co-infection (E. coli K1 + S. pneumoniae), two cases of mixed bacterial-viral coinfection (S. pneumoniae + CMV, S. agalactiae + CMV), one case of mixed yeast-viral coinfection (C. neoformans + CMV) and one case of mixed viral-viral coinfection (HHV-6 + CMV).
Any discrepancy between CSF culture and the FilmArray ME panel was analyzed in the present study. With regard to bacteria and yeast detection, the comparator targeted PCR assay and clinical data confirmed 12 of the positive results and 3 of the negative results detected by the FilmArray ME panel. For the 3 FP results, the discrepancy investigation did not support the FilmArray ME panel results: an S. agalactiae FP sample from a 1-month-old girl with normal CSF parameters; a S. pneumoniae FP sample from a 10-month-old girl, although the detection of anti-S. pneumoniae antibody was positive; and an E. coli K1 FP sample from a 26-day-old boy whose blood culture was shown to contain S. agalactiae. The false-positive results (4.4%) with the FilmArray ME panel in our study were fewer than those reported in a previous study by Leber et al. (10), in which FP results accounted for 41% of bacterial results. Only an E. coli FN case was determined in a 20-day-old boy, and the reasons for the FN results were diverse. For E. coli, only the K1 capsular type was detected by the FilmArray ME panel, while other E. coli types also cause CNS infections. In addition, frozen samples or operational issues may produce negative results (27). Clinicians should be cautious when interpreting the results from the FilmArray ME panel, particularly with regard to FP results, which may lead to needless therapy and subsequent related drug toxicity (28).
There were several limitations in our study. First, we did not have viral comparative results to support the results detected by the FilmArray ME panel because a serological test is the only major available method to detect viruses in routine diagnosis in China (29). Moreover, the serological test results were not available for some samples in our study. Although many developed countries have used real-time PCR of the CSF for the daily detection of possible viruses (30), this assay was unavailable in our study. In contrast, the FilmArray ME panel fills the gap in virological testing in China. We identified 17 positive viral pathogens including EV (n=10), CMV (n=5), HSV-1 (n=1) and HHV-6 (n=1), among which only 3 samples were positive in viral serological tests. Second, the sample size was relatively small and likely had an impact on the statistical certainty of the FilmArray ME panel sensitivity and specificity calculations. Furthermore, since it was a retrospective study, selection bias is inevitable due to the criteria used for sample selection and the small volume of CSF tested.
Conclusions
This study demonstrated the capability of the FilmArray ME panel in the diagnosis of bacterial and fungal meningitis and therefore its potential use in facilitating enhanced patient care. The BioFire FilmArray ME panel may reduce diagnostic uncertainty in pediatric patients with suspected CNS infections, and its rapid diagnosis will enable optimization of antibiotic use. However, the FilmArray ME panel cannot identify some other pathogens not included in the panel (e.g., Mycobacterium tuberculosis, an important cause of meningitis in China), nor could it provide information about antibiotic susceptibilities. Thus, it should be noted that the FilmArray ME panel represents an adjunctive test rather than a replacement test.
Acknowledgments
We would like to thank the patients and their parents for their support in permitting us to publish the findings of our research.
Funding: This work was financially supported by the Love Charity Foundation Research Project in Shanghai Children’s Medical Center (2017SCMC-AY004), the Key Developing Disciplines Project from Shanghai Municipal Commission of Health and Family Planning (2016ZB0104) and the Collaborative Innovation Center for Translational Medicine at Shanghai Jiao Tong University School of Medicine (TM201616).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board and the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB-K2017059). Written informed consent for specimen collection was obtained from the parents of the participants when lumbar puncture was carried out. But written informed consent for research could not be obtained as our study was a retrospective study.
Footnotes
Conflicts of Interest: Y Xia was employed by company bioMérieux (Shanghai) Company Limited. The other authors have no conflicts of interest to declare.
References
- 1.Dong BQ, Tang ZZ, Lin M, et al. Epidemiologic surveillance for bacterial meningitis in 140 000 children under 5 years of age in Nanning district, Guangxi province. Zhonghua Liu Xing Bing Xue Za Zhi 2004;25:391-5. [PubMed] [Google Scholar]
- 2.Li Y, Yin Z, Shao Z, et al. Population-based Surveillance for Bacterial Meningitis in China, September 2006–December 2009. Emerg Infect Dis 2014;20:61-9. 10.3201/eid2001.120375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Wang CQ, Wang XH. Etiology and antimicrobial susceptibility of children with bacterial meningitis. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-SYQK200606020.htm
- 4.Le Saux N. Guidelines for the management of suspected and confirmed bacterial meningitis in Canadian children older than one month of age. Paediatr Child Health 2014;19:141-52. 10.1093/pch/19.3.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001;108:1169-74. [PubMed] [Google Scholar]
- 6.Polage CR, Cohen SH. State-of-the-Art Microbiologic Testing for Community-acquired Meningitis and Encephalitis. J Clin Microbiol 2016;54:1197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks JT, Bharara S, Tubbs RS, et al. Polymerase chain reaction for the rapid detection of cerebrospinal fluid shunt or ventriculostomy infections. Neurosurgery 2005;57:1237-43; discussion 1237-43. 10.1227/01.NEU.0000186038.98817.72 [DOI] [PubMed] [Google Scholar]
- 8.Graf EH, Farquharson MV, Cárdenas AM. Comparative evaluation of the FilmArray meningitis/encephalitis molecular panel in a pediatric population. Diagn Microbiol Infect Dis 2017;87:92-4. 10.1016/j.diagmicrobio.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 9.Messacar K, Breazeale G, Robinson CC, et al. Potential clinical impact of the film array meningitis encephalitis panel in children with suspected central nervous system infections. Diagn Microbiol Infect Dis 2016;86:118-20. 10.1016/j.diagmicrobio.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leber AL, Everhart K, Balada-Llasat JM, et al. Multicenter Evaluation of BioFire FilmArray Meningitis/Encephalitis Panel for Detection of Bacteria, Viruses, and Yeast in Cerebrospinal Fluid Specimens. J Clin Microbiol 2016;54:2251-61. 10.1128/JCM.00730-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuti BP, Bello EO, Jegede TO, et al. Epidemiological, clinical and prognostic profile of childhood acute bacterial meningitis in a resource poor setting. J Neurosci Rural Pract 2015;6:549-57. 10.4103/0976-3147.165424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briand C, Levy C, Baumie F, et al. Outcomes of bacterial meningitis in children. Med Mal Infect 2016;46:177-87. 10.1016/j.medmal.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 13.Nigrovic LE, Fine AM, Monuteaux MC, et al. Trends in the management of viral meningitis at United States children's hospitals. Pediatrics 2013;131:670-6. 10.1542/peds.2012-3077 [DOI] [PubMed] [Google Scholar]
- 14.George BP, Schneider EB, Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000-2010. PLoS One 2014;9:e104169. 10.1371/journal.pone.0104169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soucek DK, Dumkow LE, VanLangen KM, et al. Cost Justification of the BioFire FilmArray Meningitis/Encephalitis Panel Versus Standard of Care for Diagnosing Meningitis in a Community Hospital. J Pharm Pract 2019;32:36-40. 10.1177/0897190017737697 [DOI] [PubMed] [Google Scholar]
- 16.Arora HS, Asmar BI, Salimnia H, et al. Enhanced Identification of Group B Streptococcus and Escherichia Coli in Young Infants with Meningitis Using the Biofire Filmarray Meningitis/Encephalitis Panel. Pediatr Infect Dis J 2017;36:685-7. 10.1097/INF.0000000000001551 [DOI] [PubMed] [Google Scholar]
- 17.Wootton SH, Aguilera E, Salazar L, et al. Enhancing pathogen identification in patients with meningitis and a negative Gram stain using the BioFire FilmArray(®) Meningitis/Encephalitis panel. Ann Clin Microbiol Antimicrob 2016;15:26. 10.1186/s12941-016-0137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qazi SA, Khan MA, Mughal N, et al. Dexamethasone and bacterial meningitis in Pakistan. Arch Dis Child 1996;75:482-8. 10.1136/adc.75.6.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seth R, Murthy PS, Sistla S, et al. Rapid and Accurate Diagnosis of Acute Pyogenic Meningitis Due to Streptococcus Pneumoniae, Haemophilus influenzae Type b and Neisseria meningitidis Using A Multiplex PCR Assay. J Clin Diagn Res 2017;11:FC01-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuman MI, Tolford S, Harper MB. Test characteristics and interpretation of cerebrospinal fluid gram stain in children. Pediatr Infect Dis J 2008;27:309-13. 10.1097/INF.0b013e31815f53ba [DOI] [PubMed] [Google Scholar]
- 21.Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010;23:467-92. 10.1128/CMR.00070-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afifi S, Wasfy MO, Azab MA, et al. Laboratory-based surveillance of patients with bacterial meningitis in Egypt (1998-2004). Eur J Clin Microbiol Infect Dis 2007;26:331-40. 10.1007/s10096-007-0280-x [DOI] [PubMed] [Google Scholar]
- 23.Francis BM, Gilbert GL. Survey of neonatal meningitis in Australia: 1987-1989. Med J Aust 1992;156:240-3. 10.5694/j.1326-5377.1992.tb139741.x [DOI] [PubMed] [Google Scholar]
- 24.Heath PT, Nik Yusoff NK, Baker CJ. Neonatal meningitis. Arch Dis Child Fetal Neonatal Ed 2003;88:F173-8. 10.1136/fn.88.3.F173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens JP, Eames M, Kent A, et al. Long term outcome of neonatal meningitis. Arch Dis Child Fetal Neonatal Ed 2003;88:F179-84. 10.1136/fn.88.3.F179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho HK, Lee H, Kang JH, et al. The causative organisms of bacterial meningitis in Korean children in 1996-2005. J Korean Med Sci 2010;25:895-9. 10.3346/jkms.2010.25.6.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CK, Chiu L, Yan G, et al. False negative results caused by erroneous automated result interpretation algorithm on the FilmArray 2.0 instrument. Clin Chem Lab Med 2018;56:e43-5. 10.1515/cclm-2017-0518 [DOI] [PubMed] [Google Scholar]
- 28.Gomez CA, Pinsky BA, Liu A, et al. Delayed Diagnosis of Tuberculous Meningitis Misdiagnosed as Herpes Simplex Virus-1 Encephalitis With the FilmArray Syndromic Polymerase Chain Reaction Panel. Open Forum Infect Dis 2016;4:ofw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L, Zhou M, Wang B, et al. Clinical characteristics and outcome of clinically diagnosed viral encephalitis in southwest China. Neurol Sci 2015;36:2191-7. 10.1007/s10072-015-2333-8 [DOI] [PubMed] [Google Scholar]
- 30.Mutton K, Guiver M. Laboratory techniques for human viral encephalitis diagnosis. Infect Disord Drug Targets 2011;11:206-34. 10.2174/187152611795768042 [DOI] [PubMed] [Google Scholar]


