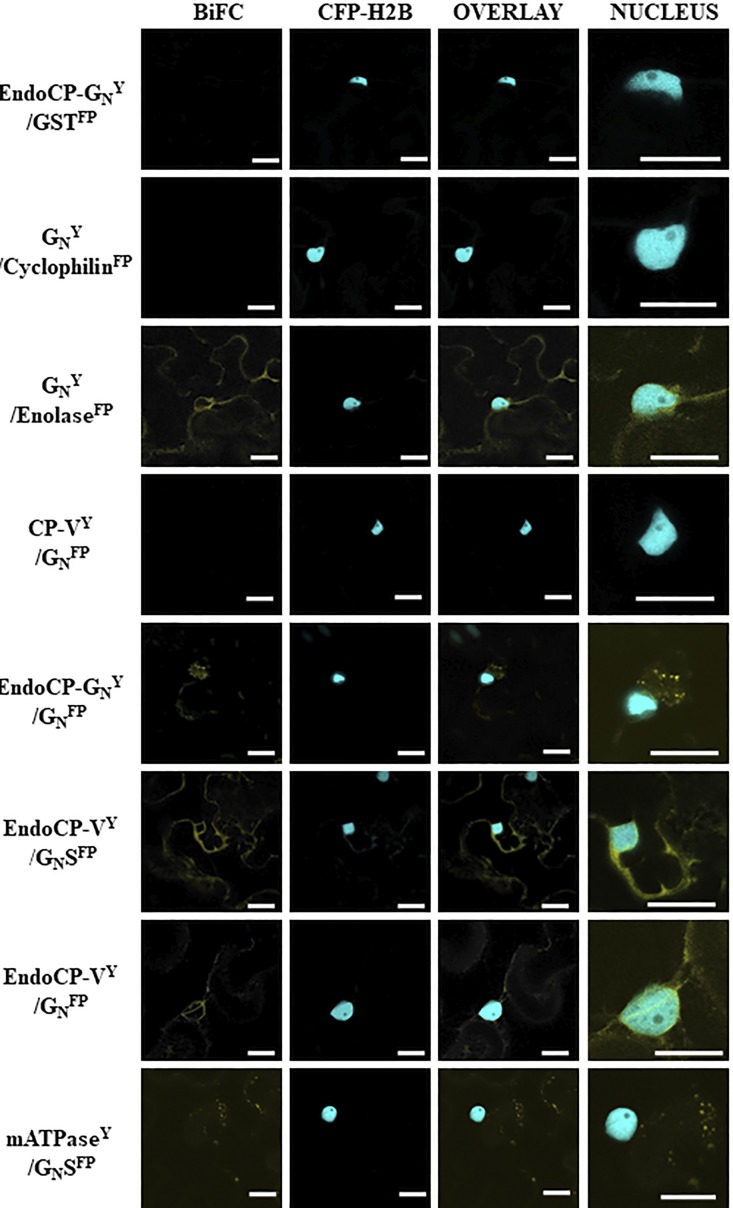
FIG 7.
Confirmation of interactions between TSWV proteins and TSWV-interacting proteins (TIPs) using bimolecular fluorescence complementation (BiFC) in Nicotiana benthamiana. Plants transgenic for a nuclear marker fused to cyan fluorescent protein (CFP-H2B) were infiltrated with suspensions of Agrobacterium tumefaciens transformed with plasmids encoding the GN protein (full-length or soluble form [GN-S]) and TIP proteins (endoCP-GN, cyclophilin, enolase, CP-V, endoCP-V, and mATPase) fused to either the amino or carboxy terminus of yellow fluorescent protein (YFP). The designation of Y indicates this is the N-terminal half of YFP, and FP represents the C-terminal half of YFP. The Y or FP position in the name indicates that all are carboxy-terminal fusions to the protein of interest. The positive interactors are seen by fluorescence of YFP in images shown in the BiFC column. The CFP-H2B column is indicated to give cellular reference, and the overlay between the two is also shown. The final column is the nucleus enlarged to show detail of the interacting TIPs within the cellular context. The first row is a representative negative control, including a TIP and glutathione S-transferase (all thrips and virus proteins were tested with the negative control to rule out nonspecific interactions). All scale bars = 20 μm.